Preparation process of lorazepam impurity D
A technology of lorazepam and preparation process is applied in the field of preparation of lorazepam impurity D, and achieves the effects of shortening preparation period, simple operation and reducing preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
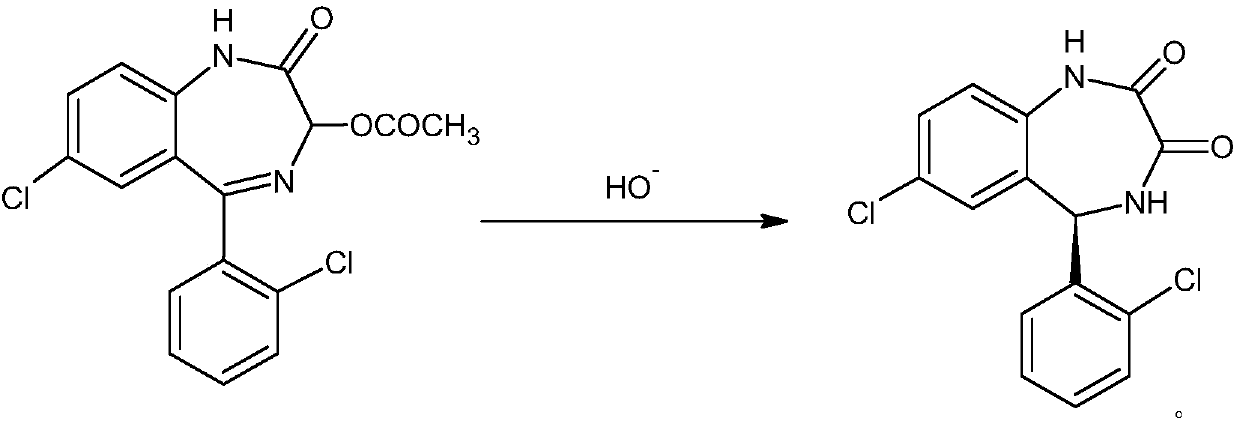
[0022] The present invention proposes a kind of preparation technology of lorazepam impurity D, comprises the steps:
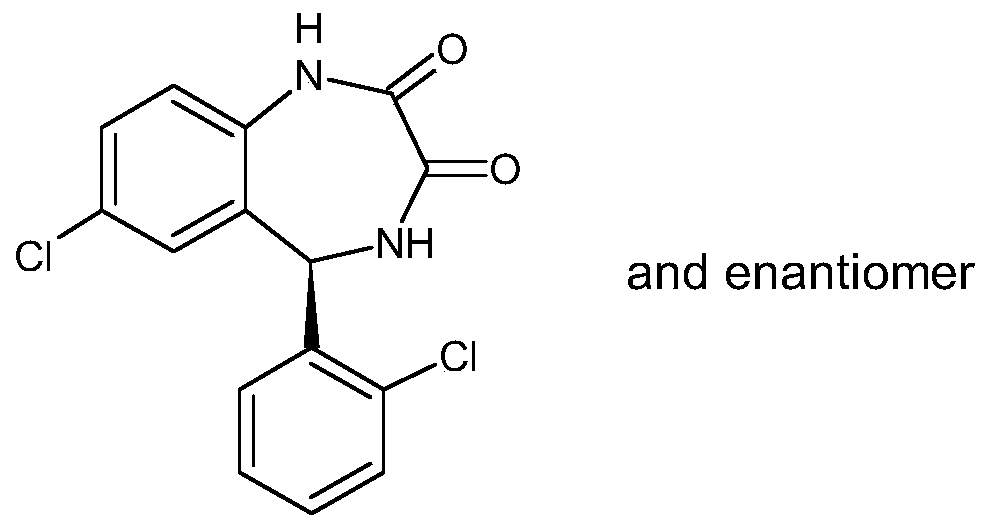
[0023] Add 350ml of methanol and 100ml of water into the reaction flask, and add 5g of potassium hydroxide under stirring until dissolved. Then add 20 g of 7-chloro-5-(2-chlorophenyl)-3-acetoxy-1,3-dihydro-2H-1,4-benzodiazepine-2-one, at 40 ℃ ~ 45 ℃ heat preservation reaction for 3 hours. Cool down to 0°C-5°C, add dropwise an aqueous solution of glacial acetic acid to neutralize to pH 6-7. Extract with 400ml×2 dichloromethane, wash neutral with water, dry over anhydrous sodium sulfate, filter out sodium sulfate, add 5g column chromatography silica gel to the filtrate and stir for 1 hour, filter column chromatography silica gel again, concentrate the filtrate under reduced pressure, Add 100ml of petroleum ether, freeze, filter, and dry to obtain 16.2g of lorazepam impurity D, with an HPLC purity of 99.89%.
Embodiment 2
[0025] The present invention proposes a kind of preparation technology of lorazepam impurity D, comprises the steps:
[0026] Add 400ml of ethanol and 100ml of water into the reaction flask, and add 5g of potassium hydroxide under stirring until dissolved. Then add 20 g of 7-chloro-5-(2-chlorophenyl)-3-acetoxy-1,3-dihydro-2H-1,4-benzodiazepine-2-one, at 55 ℃ ~ 60 ℃ heat preservation reaction for 2 hours. Cool down to 0°C-5°C, add dropwise an aqueous solution of glacial acetic acid to neutralize to pH 6-7. Extract with 400ml×2 dichloromethane, wash neutral with water, dry over anhydrous sodium sulfate, filter out sodium sulfate, add 5g column chromatography silica gel to the filtrate and stir for 1 hour, filter column chromatography silica gel again, concentrate the filtrate under reduced pressure, Add 120ml of petroleum ether, freeze, filter, and dry to obtain 16.4g of lorazepam impurity D, with an HPLC purity of 99.80%.
Embodiment 3
[0028] The present invention proposes a kind of preparation technology of lorazepam impurity D, comprises the steps:
[0029] Add 400ml of ethanol and 100ml of water into the reaction flask, and add 5g of potassium hydroxide under stirring until dissolved. Then add 20 g of 7-chloro-5-(2-chlorophenyl)-3-acetoxy-1,3-dihydro-2H-1,4-benzodiazepin-2-one at 50 ℃ ~ 55 ℃ heat preservation reaction for 2 hours. Cool down to 0°C-5°C, add dropwise an aqueous solution of glacial acetic acid to neutralize to pH 6-7. Extract with 400ml×2 ethyl acetate, wash neutral with water, dry over anhydrous sodium sulfate, filter out sodium sulfate, add 5g column chromatography silica gel to the filtrate and stir for 1 hour, filter column chromatography silica gel again, concentrate the filtrate under reduced pressure, Add 100ml of petroleum ether, freeze, filter, and dry to obtain 16.6g of lorazepam impurity D, with an HPLC purity of 99.75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com