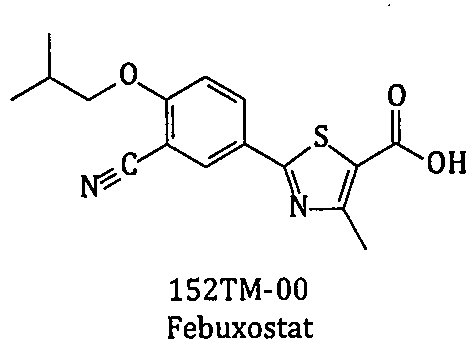
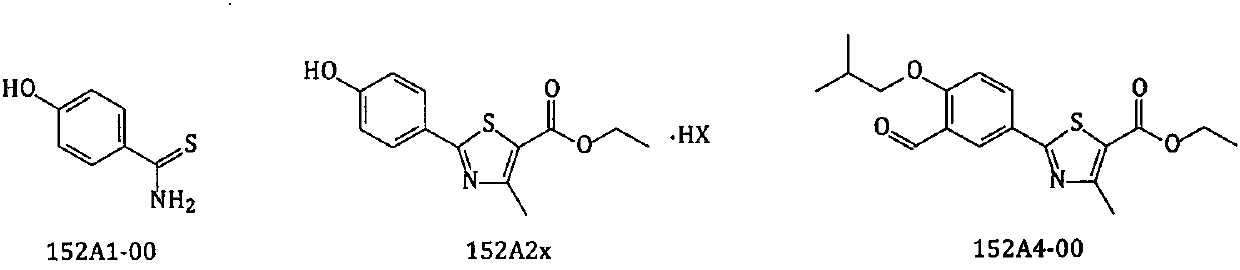
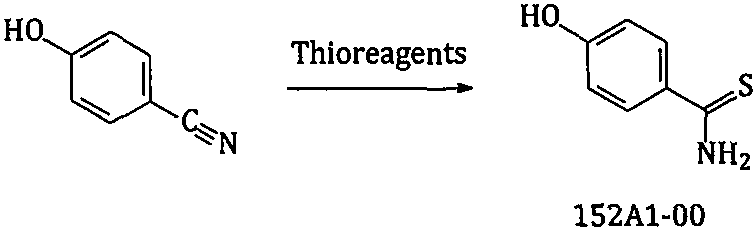
New preparation method of febuxostat intermediate
A sulfide and reagent technology, applied in the field of medicinal chemistry, can solve the problems of incomplete reaction, long time-consuming, loss of quantity, etc., and achieve the effect of reducing the amount of DMF wastewater, reducing the molar amount, and improving the utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Synthesis of 4-hydroxybenzaldehyde oxime, i.e. 152G1-00
[0048]
[0049] Add 1080g of water to the reaction flask, add 120g of methanol, add 300g (2.457mol) of 4-hydroxybenzaldehyde, add 180g (2.590mol) of hydroxylamine hydrochloride, and control the temperature not exceeding 20°C; add dropwise 102g (2.550mol) of sodium hydroxide A solution prepared with 300g of water; after the dropwise addition, keep warm at 20-25°C for 2-3 hours. After the reaction is completed, filter and collect the solid; air-dry at 55-65° C. to obtain about 318 g (theoretical amount: 336.9 g) of the dry product of 152G1-00. Yield 94.4%.
[0050] 1 H-NMR (400MHz, DMSO-d 6 ): 10.79ppm (bs, 1H,); 9.62ppm (bs, 1H); 7.96ppm (s, 1H,); 7.36ppm (d, 2H); 6.89ppm (d, 2H).
Embodiment 2
[0051] Example 2 Synthesis of 4-hydroxybenzaldehyde oxime, i.e. 152G1-00
[0052]
[0053] Add 180g methanol to the reaction flask, add 60g (491.3mmol) 4-hydroxybenzaldehyde, stir to dissolve, add 35g (503.7mmol) hydroxylamine hydrochloride, control the temperature not exceeding 30°C, add dropwise 55g (518.9mmol) anhydrous sodium carbonate A solution prepared with 500g of water; after the dropwise addition, keep warm at 20-30°C for 2-3 hours. After the reaction was completed, filter and collect the solid; air-dried at 55-65° C. to obtain about 61 g of the dry product of 152G1-00 (theoretical amount: 67.38 g). Yield 90.5%.
Embodiment 3
[0054] Embodiment 3 4-Hydroxythiobenzamide, the synthesis of 152A1-00
[0055]
[0056] Add 900g toluene to the reaction bottle; add 150g (1.094mol) 152G1-00; under stirring, add 245g (1.102mol) phosphorus pentasulfide, heat to 80-85°C, keep stirring for about 2-4hr; after the reaction is completed, cool down to 50 ~60°C, concentrate under reduced pressure to produce about 600g~700g of toluene, add 900g of water to the residue, continue to concentrate under reduced pressure to dry the remaining toluene, cool the remaining material to 5~15°C, keep warm and crystallize for about 2~4hr; filter , the filter cake was rinsed with water, and the solid was collected; air-dried at 75-85° C. to obtain about 153 g of dry product of 152A1-00 (theoretical amount: 167.6 g). Yield: 91.3%.
[0057] Take the 152A1-00 obtained above, refine it with ethanol / water, and submit the refined product for inspection 1 The H-NMR data are as follows:
[0058] 1 H-NMR (400MHz, DMSO-d 6 ): 9.98ppm ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com