Aspartate ammonia lyase mutant and application thereof
A technology of ammonia aspartate and lyase, applied in the field of enzyme catalysis, can solve the problems of low enzyme activity and low conversion rate, and achieve the effect of improving enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The construction of embodiment 1 initial aspartic acid ammonia lyase gene recombinant Escherichia coli
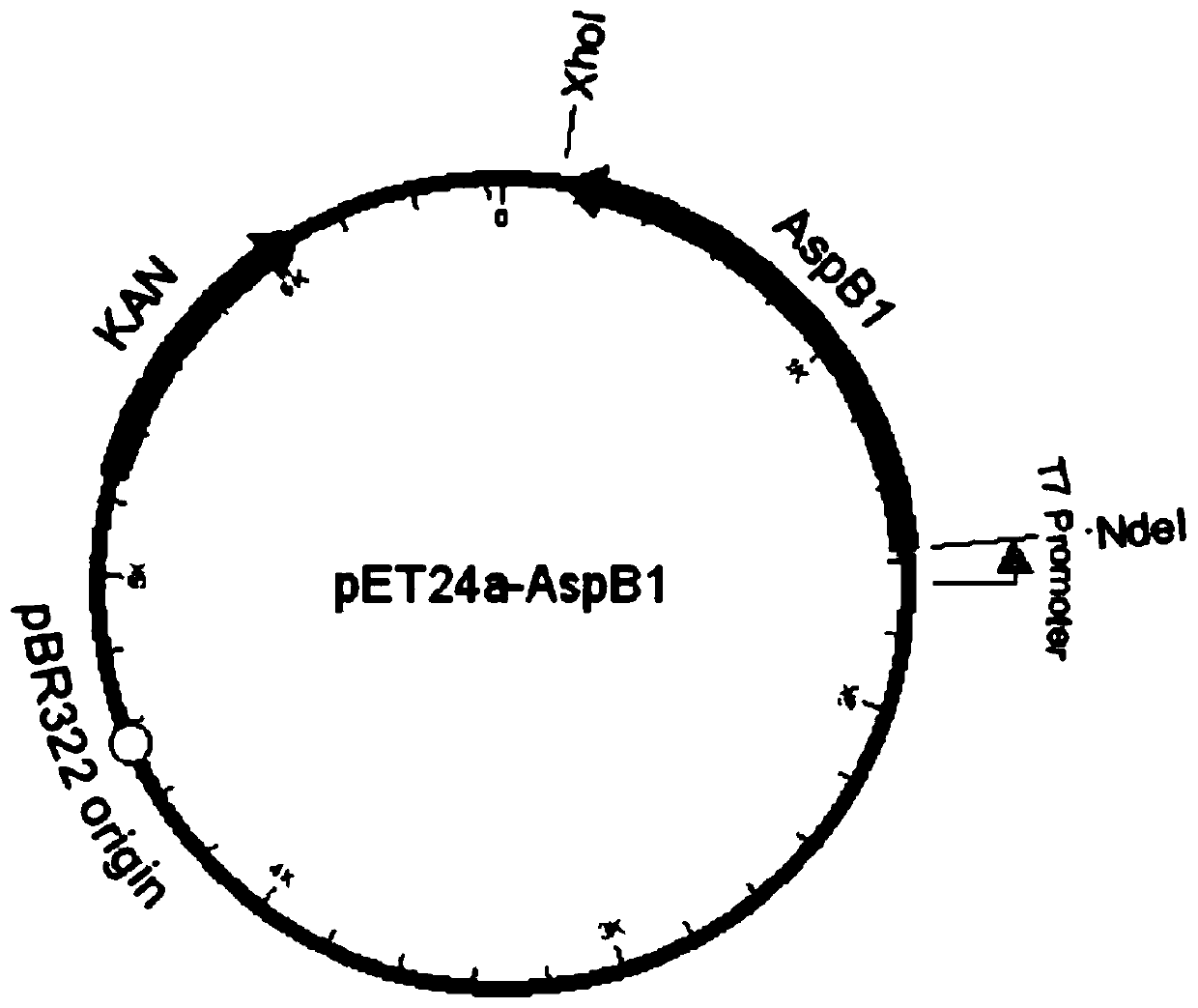
[0058] 1.1 For the aspartate ammonia lyase derived from Bacillus sp.YM55-1, according to the amino acid sequence published in the literature (RuifengLi.et al, 2018), that is, SEQ ID NO: 1, codon optimization was performed on this basis , the whole gene synthesis gene sequence SEQ ID NO: 2, and restriction endonuclease sites Nde I and XhoI were designed at both ends of the gene, subcloned into the corresponding sites of the vector pET24a (Novagen), and the recombinant plasmid pET24a-AspB1 was obtained, The plasmid map constructed see figure 1 .
[0059] 1.2 The recombinant plasmid pET24a-AspB1 was transformed into the expression host Escherichia coli BL21 (DE3) (Invitrogen Company) by electrotransformation to obtain the recombinant Escherichia coli AspB1 expressing the initial aspartate ammonia lyase.
Embodiment 2
[0060] Example 2 Construction of random mutation point library and screening by error-prone PCR method
[0061] 2.1 Error-prone PCR method to construct random mutation point library
[0062] Using the original enzyme gene SEQ ID NO: 2 as a template, an error-prone PCR technique was used to construct a random mutant library. The forward primer AspB1-F is 5’-ATGAACACCGACGTTCGTATC-3’, the reverse primer AspB1-R is 5’-TTATTTACGACCAGCGATACCCGG-3’
[0063]50μL error-prone PCR reaction system includes: 10ng plasmid template pET24a-AspB1, 50pmol pair of primers AspB1-F and AspB1-R, 1×Taq buffer, 0.2mM dGTP, 0.2mM dATP, 1mM dCTP, 1mM dTTP, 7mM MgCl 2 , (0mM, 0.05mM, 0.1mM, 0.15mM, 0.2mM) MnCl 2 , 2.5 units of Taq enzyme (fermentas company). The PCR reaction conditions were: 95°C for 5 minutes; 94°C for 30s, 55°C for 30s, 72°C for 2min / kbp; 30 cycles; 72°C for 10min. The 1.4kbp random mutation fragment was recovered from the gel as a large primer (Axygen DNA Gel Extraction Kit AP-GX...
Embodiment 3
[0073] Embodiment 3 Fermentation and transformation of mutant strain AspB-11-D4
[0074] 3.1 Shake flask fermentation
[0075] Pick a single colony from the LB culture plate of AspB-11-D4, inoculate it into 5 mL of LB liquid medium containing 50 μg / mL kanamycin sulfate, and culture overnight at 37°C and 250 rpm. Inoculate 2mL of the overnight culture into 200mL TB medium, culture at 37°C, 250rpm for 2-3h, until OD 600 At 0.6-0.8, add 0.1 mM IPTG, and culture overnight at 28°C and 200 rpm. Then at 4° C., 10000 rpm, centrifuge for 10 min, and collect the bacterial cells.
[0076] 3.2 Fermentation of initial enzyme expression strain
[0077] According to the method in step 3.1, perform shake flask fermentation on the initial enzyme expression strain AspB1, and collect the cells.
[0078] 3.3 Determination of bacterial specific activity
[0079] Add the bacterial cells AspB-11-D4 obtained in the above step 3.1 and the bacterial cells AspB1 obtained in the step 3.2 to 500 μL ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com