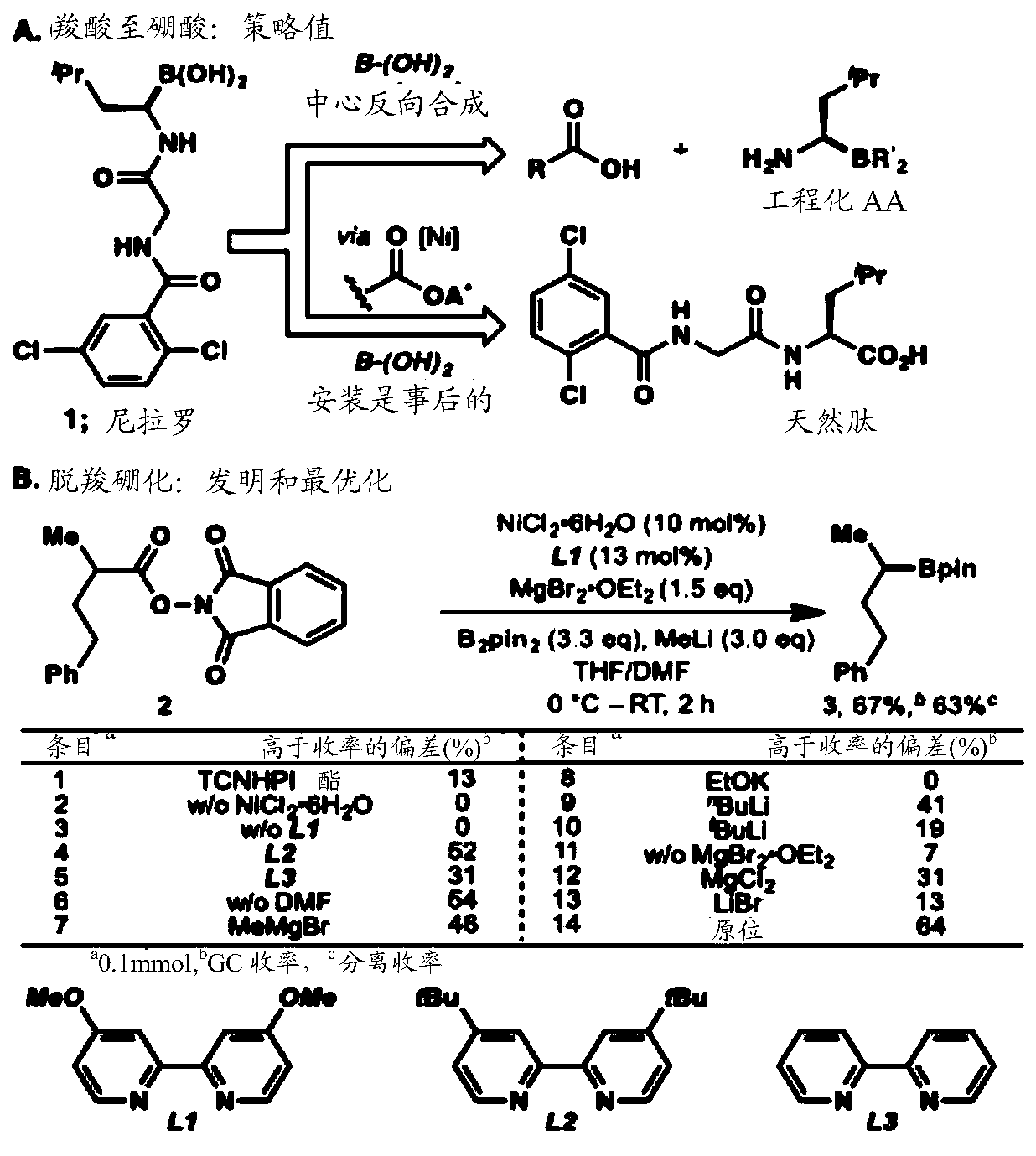
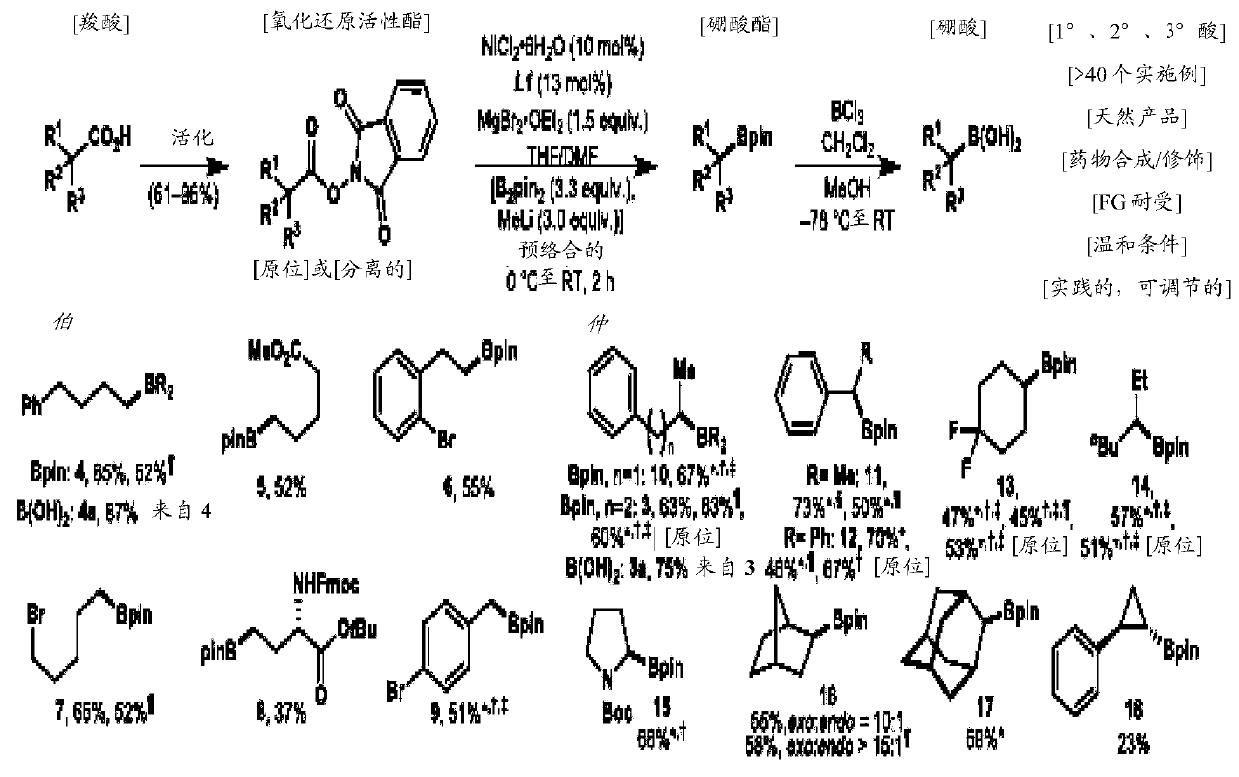
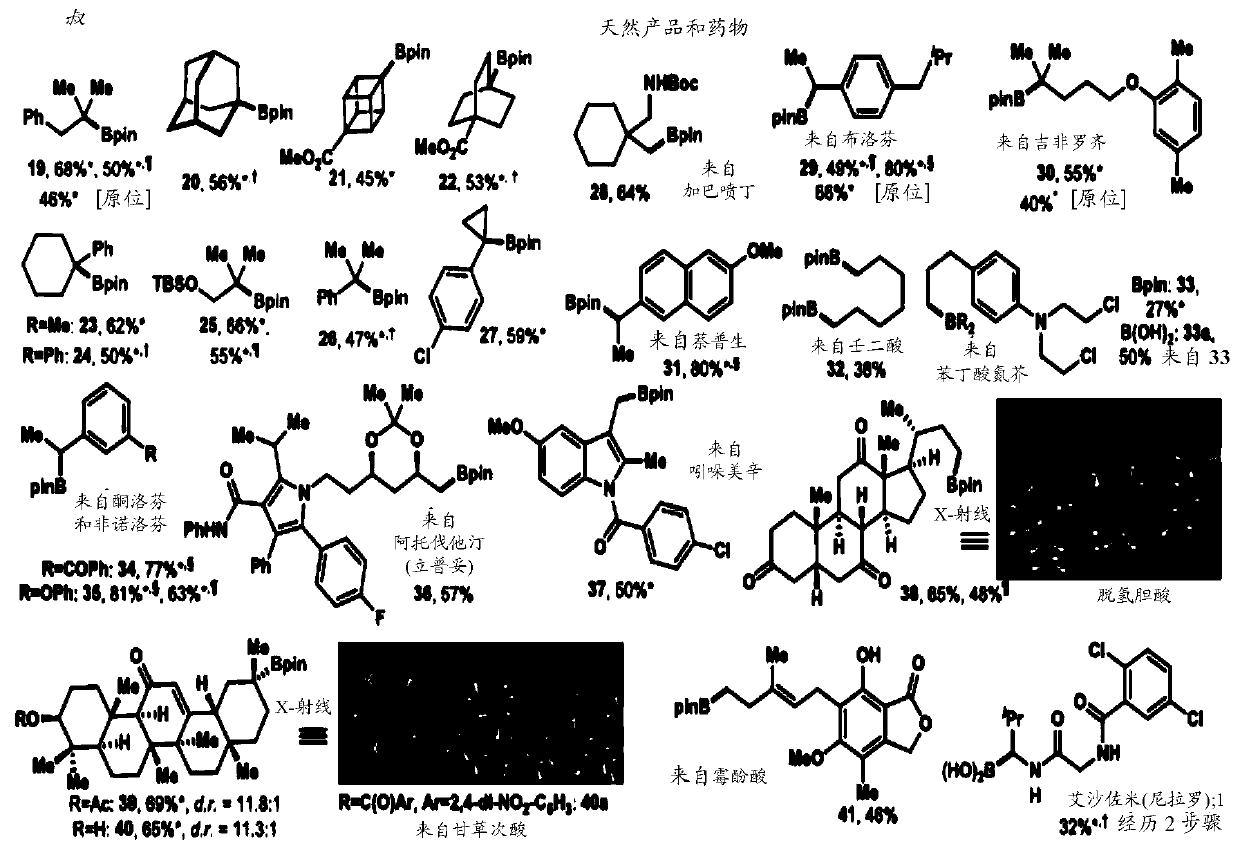
Cu-and Ni-catalyzed decarboxylative borylation reactions
A boric acid, hybrid technology, applied in catalytic reaction, boron compound active ingredient, boron compound active ingredient, etc., to achieve the effect of high chemical selectivity and cheap reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0142] [B 2 pin 2 Preparation of Me]Li complexes:
[0143] to B at 0°C under argon 2 pin 2 (1.1 eq.) in THF (B 2 pin 2 The concentration is 1.1M) to add MeLi (1.6M, in Et 2 O, 1.0 equiv). The reaction mixture was warmed to room temperature and stirred for 1 h to give a milky white suspension.
[0144] Nickel-catalyzed decarboxylation borylation:
[0145] Will be loaded with redox active ester (1.0 equivalent) and MgBr 2 ·OEt 2 (1.5 equivalents) of the flask was evacuated and backfilled three times with argon. Catalyst solution or suspension (containing 10 mol% NiCl 2 ·6H 2 O and 13 mol% ligand). When using a catalyst suspension / solution in DMF, add another portion of THF to the reaction vessel (twice the volume of the DMF suspension / solution required) followed by the catalyst mixture [this process can be quite exothermic. Cooling (with ice / water bath) may be necessary]. The resulting mixture was stirred vigorously until no visible solids were observed at the bot...
Embodiment
[0217] Boronic acid inhibitors of human neutrophil elastase prepared by the method of the invention
[0218] Disclosed compound (IC of human neutrophil elastase 50 )
[0219]
[0220] Synthetic pathway of boronic acid inhibitor of human neutrophil elastase
[0221]
[0222]
[0223] All three compounds (mCBK319, mCBK320 and mCBK323) were found to have a high tendency to trimerize in the boronic acid motif. The biological activity of these trimers may require further study. These novel elastase inhibitors have potential applications in the treatment of cancer, circulatory fibrosis and bronchiectasis.
[0224] These compounds showed higher potency (100-1000-fold increase compared to similar trifluoroquinoline lead compounds) than the lead compounds screened as elastase inhibitors. The unique physicochemical properties of the boronic acid motif can lead to favorable pharmacokinetic properties. The preparation methods disclosed herein are straightforward and easy to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com