Spinal dura mater sealing hydrogel and preparation method and application thereof
A hydrogel sealing and mixture technology, applied in the field of medical devices, can solve the problems of nerve tissue degeneration, cytotoxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The preparation method of the sealing hydrogel for preventing cerebrospinal fluid leakage of the present invention is as follows:
[0049] 0.2 g of human serum albumin (20%, w / v) was weighed and dissolved in 1 mL of phosphate buffer (10 mM, pH=9.2) prepared from deionized water. Stir intermittently at 37°C for 1 hour to dissolve the protein. After the protein is completely dissolved and becomes a transparent liquid, use a vacuum pump for 30 minutes to quickly eliminate the bubbles in the solution.
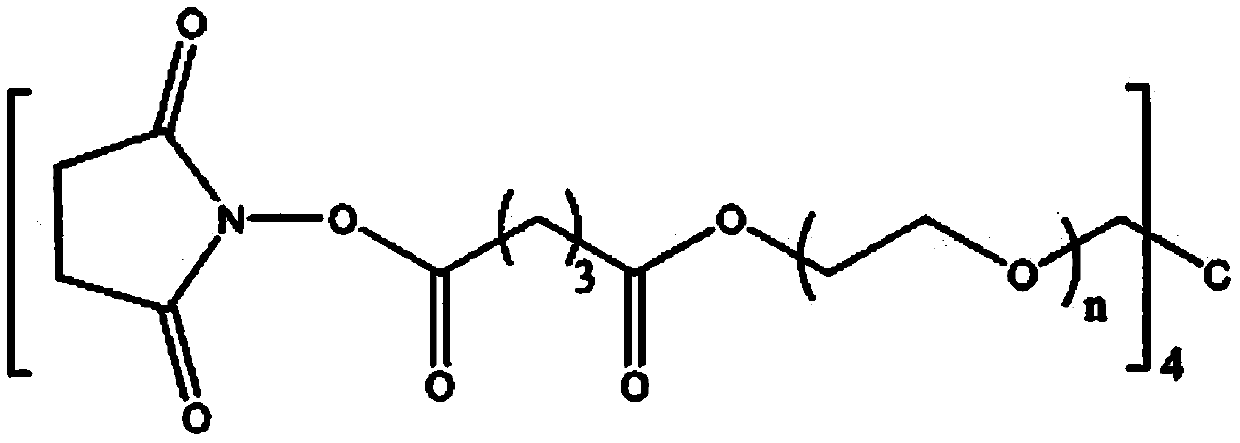
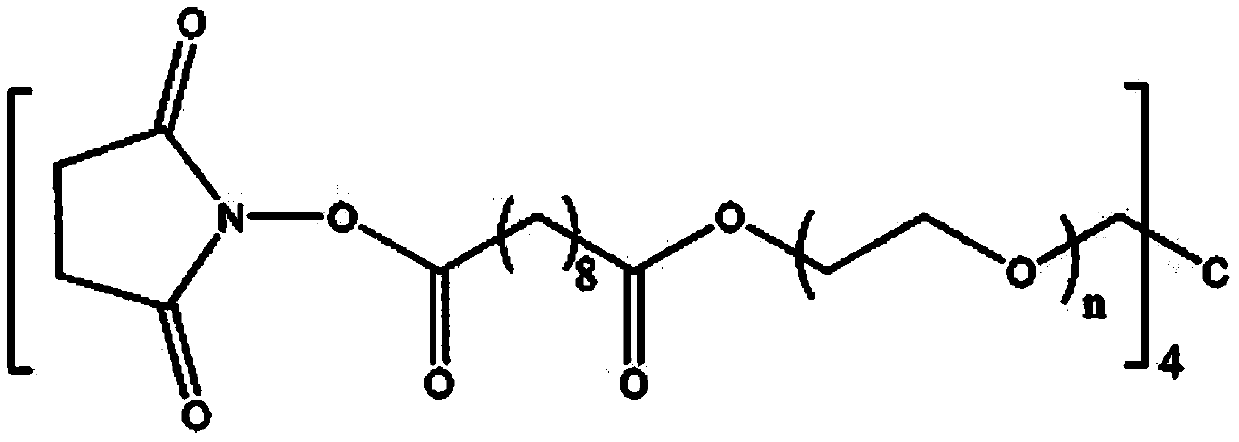
[0050] Weigh 0.2 g of 4-arm polyethylene glycol succinimide glutarate (4-arm-PEG-SG, molecular weight 20,000 Da), dissolve it in 1 mL of phosphate buffer (10 mM, pH= 7.4), vortex to dissolve into a transparent liquid mixture.
[0051] The human serum albumin solution and the 4-arm-PEG-SG solution were respectively filled into the double-drug mixer, sprayed and then cross-linked to form a hydrogel sealant. The gelling time after mixing is 3 seconds, the swelling degree of t...
Embodiment 2
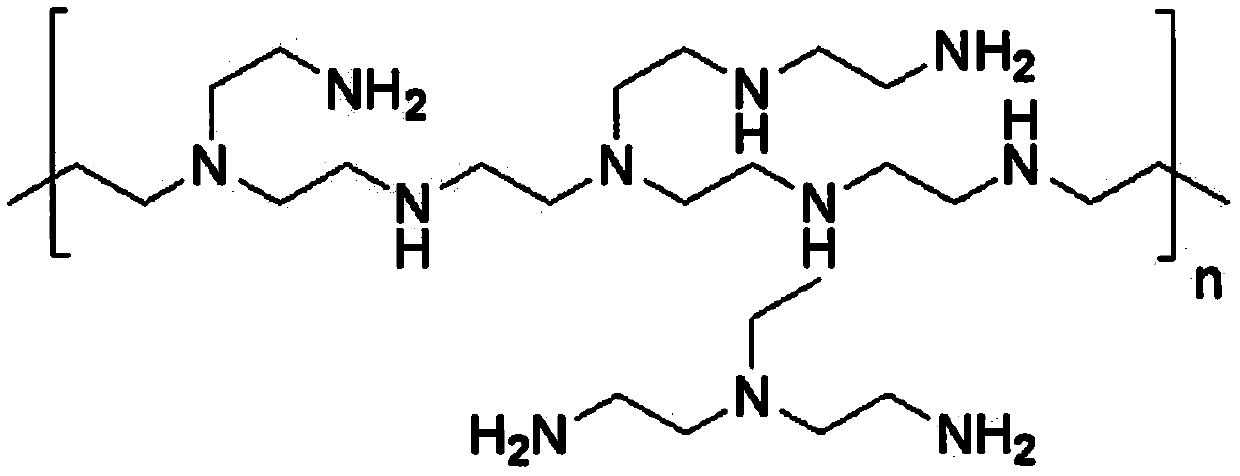
[0053] 0.2 g of human serum albumin and 0.0135 g of hyperbranched polyethyleneimine were weighed and dissolved in 1 mL of phosphate buffer (10 mM, pH=9.2) prepared from deionized water. Stir intermittently at 37°C for 1 hour to dissolve the protein. After the protein is completely dissolved and becomes a transparent liquid, use a vacuum pump for 30 minutes to quickly eliminate the bubbles in the solution.
[0054] Weigh 0.2 g of 4-arm polyethylene glycol succinimide glutarate (4-arm-PEG-SG, molecular weight 20,000 Da), dissolve it in 1 mL of phosphate buffer (10 mM, pH= 7.4), vortex to dissolve into a transparent liquid mixture.
[0055] The human serum albumin solution and the 4-arm-PEG-SG solution were respectively filled into the double-drug mixer, sprayed and then cross-linked to form a hydrogel sealant. The gelling time after mixing is 2 seconds, the swelling degree of the prepared serum albumin gel is 126%, the bursting strength reaches 26.8kPa, and the degradation time...
Embodiment 3
[0057]0.2 g of human serum albumin and 0.0135 g of hyperbranched polyethyleneimine were weighed and dissolved in 1 mL of phosphate buffer (10 mM, pH=9.2) prepared from deionized water. Stir intermittently at 37°C for 1 hour to dissolve the protein. After the protein is completely dissolved and becomes a transparent liquid, use a vacuum pump to pump air for 30 minutes to quickly eliminate the bubbles in the solution.
[0058] Weigh 0.2 g of 4-arm polyethylene glycol ether tetrasuccinimide sebacate (4-arm-PEG-SSeb, molecular weight 12000D), dissolve it in 1 ml of phosphate buffer (10 mM, pH=6.0), vortex to dissolve into a transparent liquid mixture.
[0059] Plasma-derived human serum albumin solution and 4-arm-PEG-SSeb solution were respectively filled into a double-drug mixer, sprayed and then cross-linked to form serum albumin gel. The gelling time after mixing is 18 seconds, the swelling degree of the prepared serum albumin gel is 108%, the bursting strength reaches 34.2kPa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bursting strength | aaaaa | aaaaa |
| Bursting strength | aaaaa | aaaaa |
| Bursting strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com