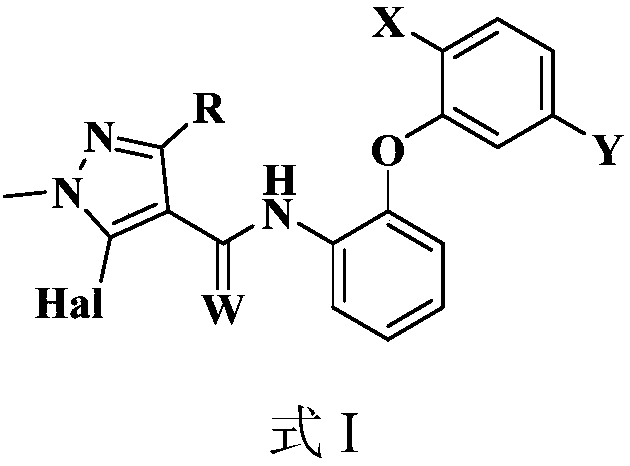
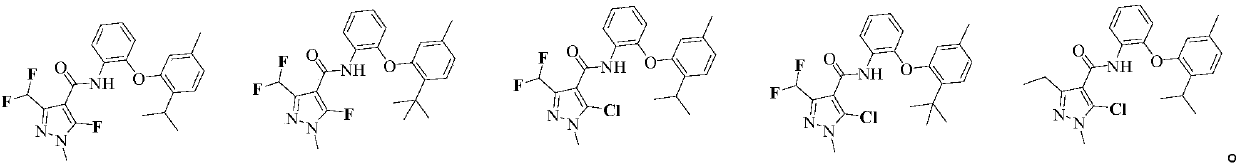
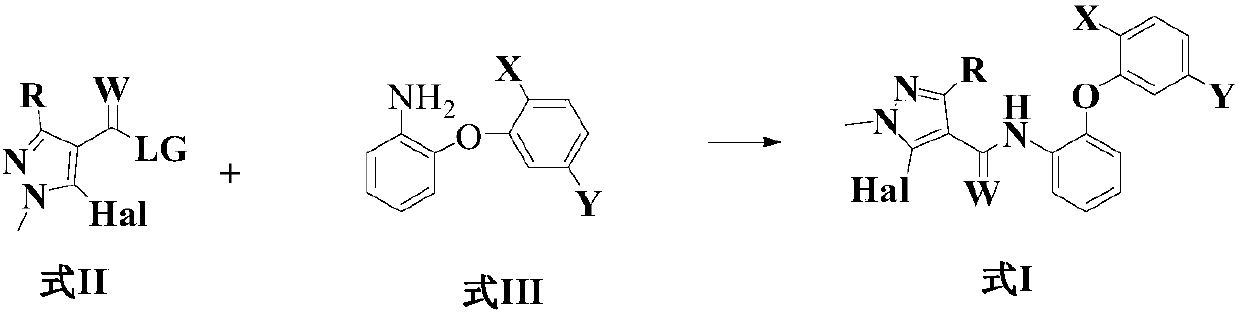
Amide compound and preparation method and application thereof
A technology of amide compounds and compounds, applied in the direction of botany equipment and methods, applications, biocides, etc., can solve problems such as poor results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] N-(2-(2-异丙基-5-甲基苯氧基)苯基)-1-甲基-3-二氟甲基-5-氟-1H-吡唑-4-甲酰胺(化合物1)的制备:
[0087] 步骤1:1-异丙基-4-甲基-2-(2-硝基苯氧基)苯的合成:
[0088]
[0089] 取一反应瓶,将邻氟硝基苯(来自商业购买,70.87mmol,1.0eq)、碳酸钾(70.87mmol,1.0eq)加入到60mL DMF中,回流条件下加入2-异丙基-5-甲基苯酚(来自商业购买,77.96mmol,1.1eq),保持温度反应2-3h,待反应完毕后,加水200mL,乙酸乙酯萃取,有机层经饱和食盐水洗涤、无水硫酸钠干燥后,减压下浓缩,得到淡黄色油状粗产物1-异丙基-4-甲基-2-(2-硝基苯氧基)苯(11.9g,收率62%),未经分离,直接进行下一步反应。
[0090] 步骤2:2-(2-异丙基-5-甲基苯氧基)苯胺的合成:
[0091]
[0092]Add 1-isopropyl-4-methyl-2-(2-nitrophenoxy)benzene (43.86mmol, 1.0eq), ethanol (60mL), 5% palladium on carbon (1.19g) to the reaction flask successively , hydrazine hydrate (175.44mmol, 4eq) was slowly added dropwise to the above system, and reacted at room temperature for 4h. After the reaction was completed, the palladium carbon was removed by filtration, water was added to the filtrate, extracted with ethyl acetate, and the organic layer was washed with saturated brine, After drying over anhydrous sodium sulfate, it was concentrated under reduced pressure...
Embodiment 3
[0103] N-(2-(2-tert-butyl-5-methylphenoxy)phenyl)-1-methyl-3-difluoromethyl-5-fluoro-1H-pyrazole-4-carboxamide ( Compound 2) Preparation:
[0104] Step 1: Synthesis of 1-tert-butyl-4-methyl-2-(2-nitrophenoxy)benzene:
[0105]
[0106] Take a reaction bottle, dissolve o-fluoronitrobenzene (from commercial purchase, 70.87mmol, 1.0eq), potassium carbonate (70.87mmol, 1.0eq) in 60mL DMF, add 2-(tert-butyl)-5 -Methylphenol (from commercial purchase, 77.96mmol, 1.1eq), keep the temperature and react for 2-3h, after the reaction is completed, add 200mL of water, extract with ethyl acetate, and the organic layer is washed with saturated brine and dried over anhydrous sodium sulfate Afterwards, it was concentrated under reduced pressure to obtain a brown oily crude product 1-tert-butyl-4-methyl-2-(2-nitrophenoxy)benzene (18.2 g, yield 90%).
[0107] Step 2: Synthesis of 2-(2-tert-butyl-5-methylphenoxy)aniline:
[0108]
[0109] Add 1-tert-butyl-4-methyl-2-(2-nitrophenoxy)benze...
Embodiment 4
[0115] N-(2-(2-tert-butyl-5-methylphenoxy)phenyl)-1-methyl-3-difluoromethyl-5-chloro-1H-pyrazole-4-carboxamide ( Compound 38) Preparation:
[0116]
[0117] Add 2-(2-tert-butyl-5-methylphenoxy)aniline (1.96mmol, 1.0eq), dichloromethane (20mL), triethylamine (2.08mmol, 1.06eq) successively to the reaction flask, 1-Methyl-3-difluoromethyl-5-chloro-1H-pyrazole-4-carbonyl chloride (2.06mmol, 1.05eq) was slowly added dropwise. React at room temperature for 2 h, add water to the reaction solution to quench the reaction, extract with dichloromethane, wash the organic layer with saturated brine, dry over anhydrous sodium sulfate, concentrate under reduced pressure, and purify the residue by column chromatography (eluent Petroleum ether: ethyl acetate = 4:1), the off-white solid product N-(2-(2-tert-butyl-5-methylphenoxy)phenyl)-1-methyl-3-difluoro Methyl-5-chloro-1H-pyrazole-4-carboxamide (600 mg, yield 68.3%).
[0118] Compound 38 1 H NMR (400MHz, DMSO) data are as follows (δ[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com