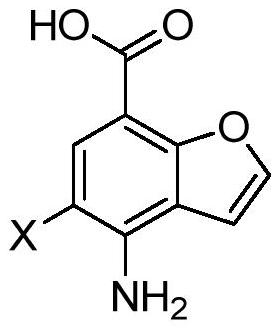
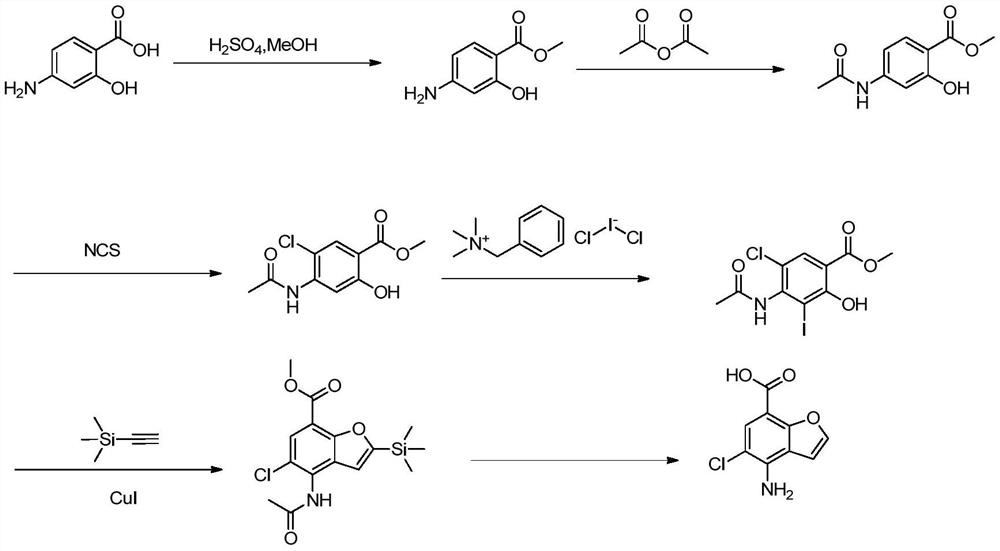
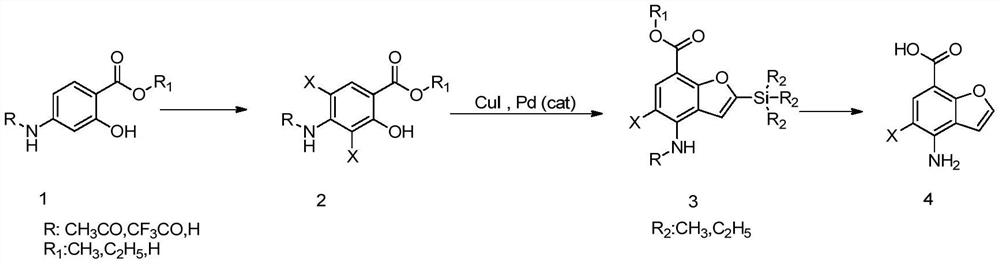
Preparation method of key intermediate 4-amino-5-halobenzofuran-7-carboxylic acid of 5-ht4 receptor agonist
A technology of hydroxybenzoic acid and amino, which is applied in organic chemistry, bulk chemical production, etc., can solve problems such as difficult purification, complicated operation, and low yield of synthesis process, so as to improve market competitiveness, optimize preparation process, and process heavy good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The first step: the synthesis of 4-amino-3,5-dibromo-2-hydroxybenzoic acid
[0038] Dissolve p-amino-o-hydroxybenzoic acid (146.3g, 0.956mol, 1.0eq) in dichloroethane (4.0L), add NBS (373.8g, 2.1mol, 2.2eq) under stirring, and heat to 80-85 ℃, after 4 hours, the central control, HPLC showed that the raw material was completely consumed, the temperature was lowered to below 35 ℃, and the solvent was evaporated under reduced pressure. Pour the system into 2.0L ice water, stir vigorously for half an hour, filter, wash twice with water to obtain the crude product, beat the crude product with ethanol twice, drain, and recrystallize the filter cake with ethyl acetate to obtain 4-amino-3,5-dibromo - 274.5 g of 2-hydroxybenzoic acid, purity 99%, yield: 93.0%. m / z=311.7[MH] detected by mass spectrometry + , and the solid was determined to be 4-amino-3,5-dibromo-2-hydroxybenzoic acid.
[0039] The second step: Synthesis of 2-trimethylsilyl-4-amino-5-bromobenzofuran-7-carboxyli...
Embodiment 2
[0045] Step 1: Synthesis of 4-amino-3,5-dichloro-2-hydroxybenzoic acid
[0046] Dissolve p-amino-o-hydroxybenzoic acid (146.3g, 0.956mol, 1eq) in dioxane (4.0L), add NCS (333.8g, 2.5mol, 2.6eq) under stirring, and heat to 80-85°C , after 4 hours of central control, HPLC showed that the raw materials were completely consumed, the temperature was lowered to below 35°C, and the solvent was evaporated under reduced pressure. The system was poured into 2.0L ice water, stirred vigorously for half an hour, filtered, washed twice with water to obtain the crude product, the crude product was beaten with ethanol twice, drained, and the filter cake was recrystallized with ethyl acetate to obtain 4-amino-3,5-dichloro -2-Hydroxybenzoic acid 198.5g, purity 99%, yield: 94%. Mass spectrometry m / z=222.2[MH] + , and the solid was determined to be 4-amino-3,5-dichloro-2-hydroxybenzoic acid.
[0047] The second step: the synthesis of 2-triethylsilyl-4-amino-5-chlorobenzofuran-7-carboxylic acid...
Embodiment 3
[0053] The first step: the synthesis of methyl 4-acetamido-3,5-dichloro-2-hydroxybenzoate
[0054] Dissolve methyl p-acetamido-o-hydroxybenzoate (200g, 0.956mol, 1.0eq) in acetonitrile (4.0L), add dichlorohydantoin (376.3g, 1.91mol, 2.0eq) under stirring, and heat to 80 -85°C, central control after 4 hours, HPLC showed that the raw materials were completely consumed, the temperature was lowered to below 35°C, and the solvent was evaporated under reduced pressure. The system was poured into 2.0L ice water, stirred vigorously for half an hour, filtered, washed twice with water to obtain the crude product, the crude product was beaten twice with ethanol, drained, and the filter cake was recrystallized with ethyl acetate to obtain 4-acetylamino-3,5-di Chloro-2-hydroxybenzoic acid methyl ester 243.6g, purity 99%, yield: 92%. Mass spectrometry m / z=278.1[MH] + , identified as methyl 4-acetamido-3,5-dichloro-2-hydroxybenzoate.
[0055] The second step: Synthesis of methyl 2-trimeth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com