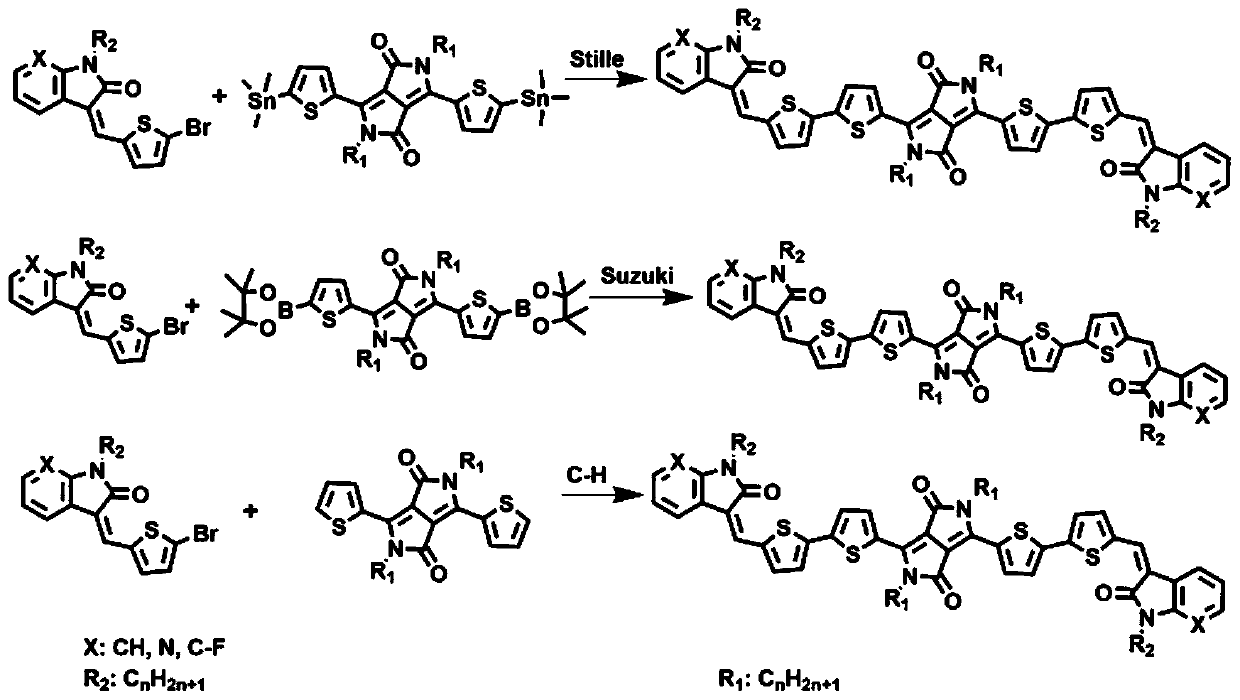
Solution-processible conjugated micro-molecular semiconductor materials based on thiophene pyrrolopyrroledione and semi-indigo
A technology of thiophene diketopyrrolopyrrole and diketopyrrolopyrrole, which is applied in the field of A2-D-A1-D-A2 type conjugated small molecule semiconductor materials, can solve the problems of poor film-forming properties of small molecules and achieve strong The effect of interaction, excellent solubility, and excellent photoelectric performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
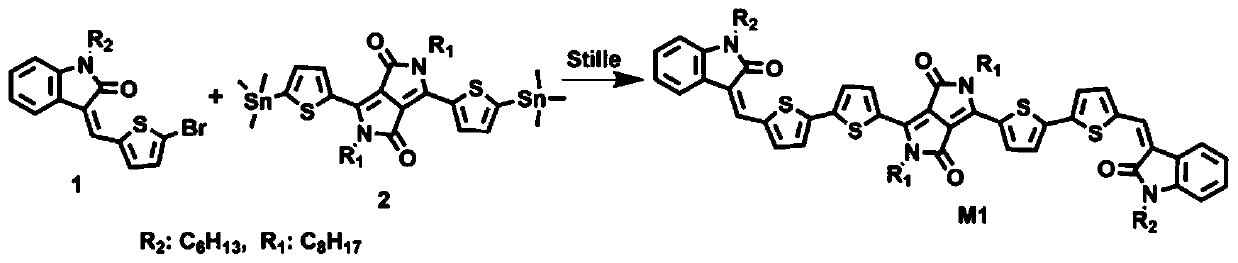
[0021] Embodiment 1: synthesis A2-D-A1-D-A2 type conjugated small molecule semiconductor material M1 (such as figure 2 shown),
[0022] Step 1, Synthesize monomer 1 of conjugated small molecule semiconductor material M1 with reference to literature ((Z)-(Thienylmethylene)oxindole-Based Polymers for High Performance SolarCells.Macromolecules.2016,49,2145-2152.) Synthesis, monomer 2 It can be purchased commercially. The stille reaction is used to synthesize the conjugated small molecule semiconductor material M1, and the brominated monomer 1 (0.063g, 0.162mmol) and the double tinned monomer 2 (0.06g, 0.071mmol) are respectively added to the Schlenk bottle. Solvent toluene (10mL), nitrogen replacement for 20 minutes, then add catalyst Pd 2 (dba) 3 (3mg), Ligand P(o-tol) 3 (4 mg), reacted at 110° C. for 24 hours, cooled to room temperature, added water, extracted with dichloromethane, collected the organic layer, dried, removed the organic solvent, and purified by column chrom...
Embodiment 2
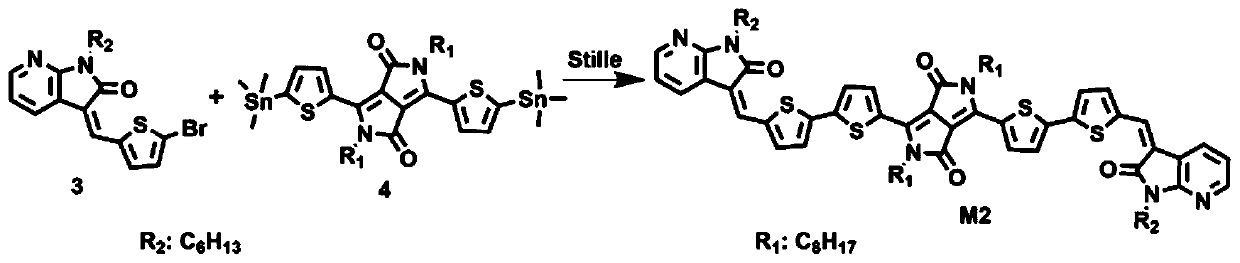
[0023] Embodiment 2: synthesis A2-D-A1-D-A2 type conjugated small molecule semiconductor material M2 (such as image 3 shown)
[0024] Step 2, synthesize the monomer 3 of the conjugated small molecule semiconductor material M2, refer to the literature ((Z)-(Thienylmethylene)oxindole-Based Polymers for High Performance SolarCells.Macromolecules.2016,49,2145-2152.) Synthesis, monomer 2 It can be purchased commercially. The Stille reaction is used to synthesize the conjugated small molecule semiconductor material M2. Other operating methods are the same as in Example 1. Brominated monomer 3 (0.061g, 0.155mmol) and double tinned monomer 4 are respectively added to the Schlenk bottle. (0.06g, 0.071mmol), solvent toluene (10mL), nitrogen displacement 20 minutes, then add catalyst Pd 2 (dba) 3 (3mg), Ligand P(o-tol) 3 (4 mg), reacted at 110° C. for 24 hours, cooled to room temperature, added water, extracted with dichloromethane, collected the organic layer, dried, removed the org...
Embodiment 3
[0025] Embodiment 3: synthesis A2-D-A1-D-A2 type conjugated small molecule semiconductor material M3 (such as Figure 4 shown)
[0026] Step 3, synthesize monomer 5 of the conjugated small molecule semiconductor material M2 by referring to the literature ((Z)-(Thienylmethylene)oxindole-Based Polymers for High Performance SolarCells.Macromolecules.2016,49,2145-2152.) synthesis, monomer 6 It can be purchased commercially. M3 can be synthesized by the Stille method. Specifically, with reference to Example 1, the Suzuki method is used for synthesis. In the Schlenk bottle, brominated monomer 5 (0.116g, 283mmol) and boroester monomer 6 (0.1g , 0.129mmol), solvent toluene (10mL), sodium carbonate aqueous solution (2M, 2mL), nitrogen replacement for 20 minutes, then add catalyst Pd 2 (dba) 3 (3mg), Ligand P(o-tol) 3 (4 mg), stirred rapidly, reacted at 110°C for 24 hours, cooled to room temperature, added water, extracted with dichloromethane, collected the organic layer, dried, rem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com