Affinity peptide and application thereof
An affinity peptide, affinity technology, applied in the direction of peptides, specific peptides, peptide preparation methods, etc., can solve the problem of unsatisfactory separation methods and media, inability to effectively separate κ-type antibodies and λ-type antibodies, and easy inactivation. and other problems, to achieve the effect of cheap and easy-to-obtain quality control, easy media preparation, and easy quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: the synthesis of polypeptide
[0020] 1) Activated resin: Weigh 1000mg of Fmoc Cys-wang Resin, soak in N,N-dimethylformamide (DMF) for 30min to swell.
[0021] 2) Deprotection: remove DMF by pressure filtration, add the DMF solution containing 20% piperidine and blow with nitrogen to react for 15min, and remove by pressure filtration; wash the resin three times with isopropanol, wash three times with DMF, and then use the ninhydrin method to detect the resin Fmoc Remove status.
[0022] 3) Condensation reaction: Weigh 1.4mmol FMOC-Tyr, add O-benzotriazole-N,N,N',N'-tetramethyluronium tetrafluoroboric acid (TBTU), 1-hydroxybenzotriazole The DMF mixed solution of oxazole (HOBt) and N,N-diisopropylethylamine (DIEA) was used as the reaction solution, and the reaction was carried out under nitrogen blowing at room temperature for 2 hours; after the reaction, the resin was washed three times with isopropanol, and then washed with DMF Three times, detected by...
Embodiment 2
[0026] Embodiment 2: the preparation of affinity medium
[0027] 1) Dissolve 10 mg of antibody affinity peptide (amino acid sequence shown in SEQ ID No.1) in 10 mL of 4-hydroxyethylpiperazineethanesulfonic acid (Hepes) buffer, pH 7.4.
[0028] 2) Take 1 mL of Sephadex activated by sulfhydryl groups.
[0029] 3) The compounds in step 1) and 2) were mixed and reacted for 12 hours.
[0030] 4) After the reaction is completed, the gel particles are washed with Hepes buffer to obtain an affinity medium.
Embodiment 3
[0031] Example 3: Affinity peptide separation and purification of kappa chain antibody
[0032] 1) Prepare 10mmol / L Hepes buffer, pH 7.0, NaCl content 0.15M, set flow rate 1mL / min.
[0033] 2) Using the liquid prepared in step 1) as the mobile phase, run the AKTA protein purification system.
[0034] 3) Take 1 mL of the affinity medium and 1 mL of the Protein L medium in Example 2 to fill the separation column respectively.
[0035] 4) Add 1 mL of antibody solution and collect 3 mL of the effluent.
[0036] 5) Prepare 0.1M HCl-Gly buffer at pH 2 as the eluent.
[0037] 6) Load 3 mL of the eluate and collect 3 mL of the eluate.
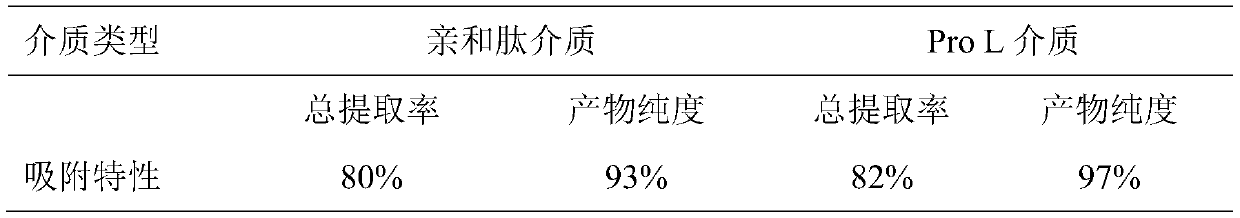
[0038] 7) Measure the content and purity of the kappa chain antibody in the effluent and eluent before adsorption, after adsorption, respectively, and calculate the total extraction rate. The results are shown in Table 1 below.
[0039] Table 1. Comparison of adsorption characteristics between affinity peptide medium and Protein L medium
[0040] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com