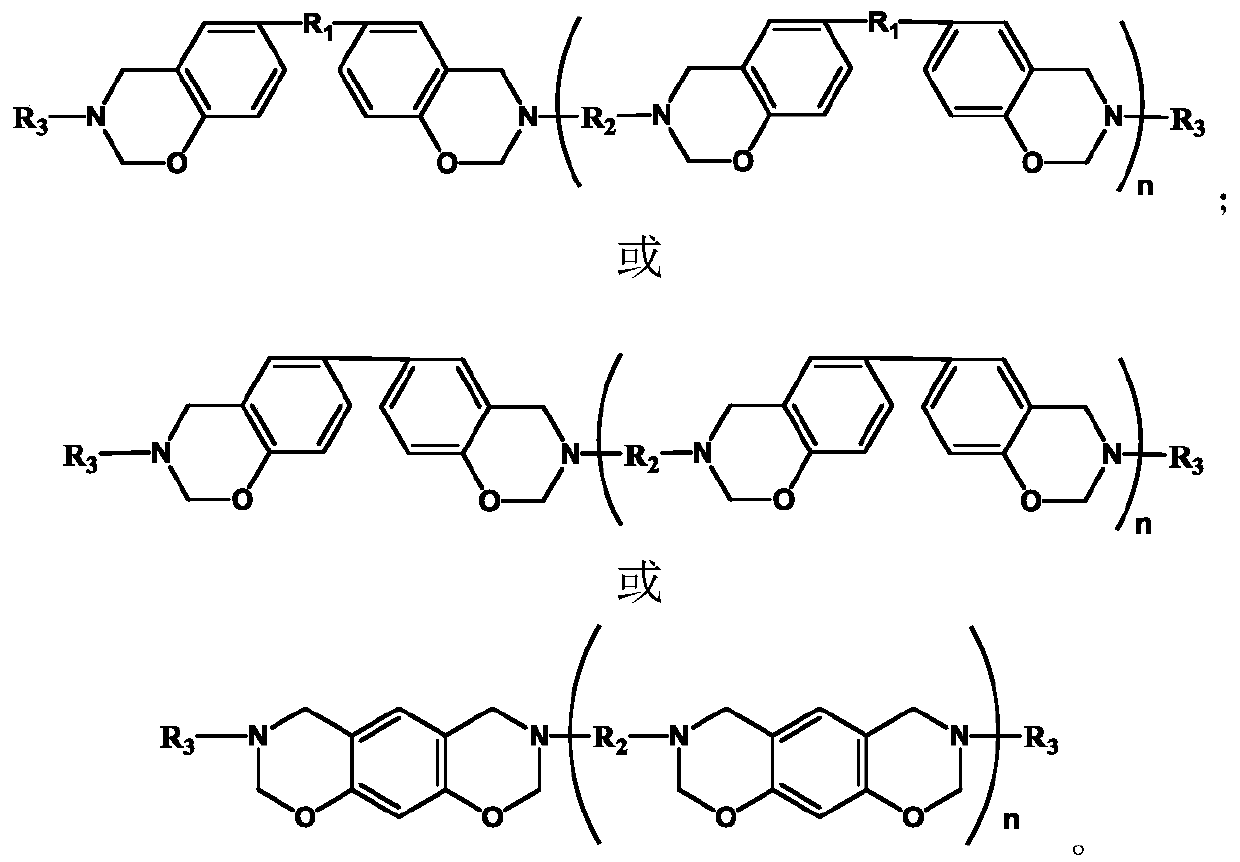
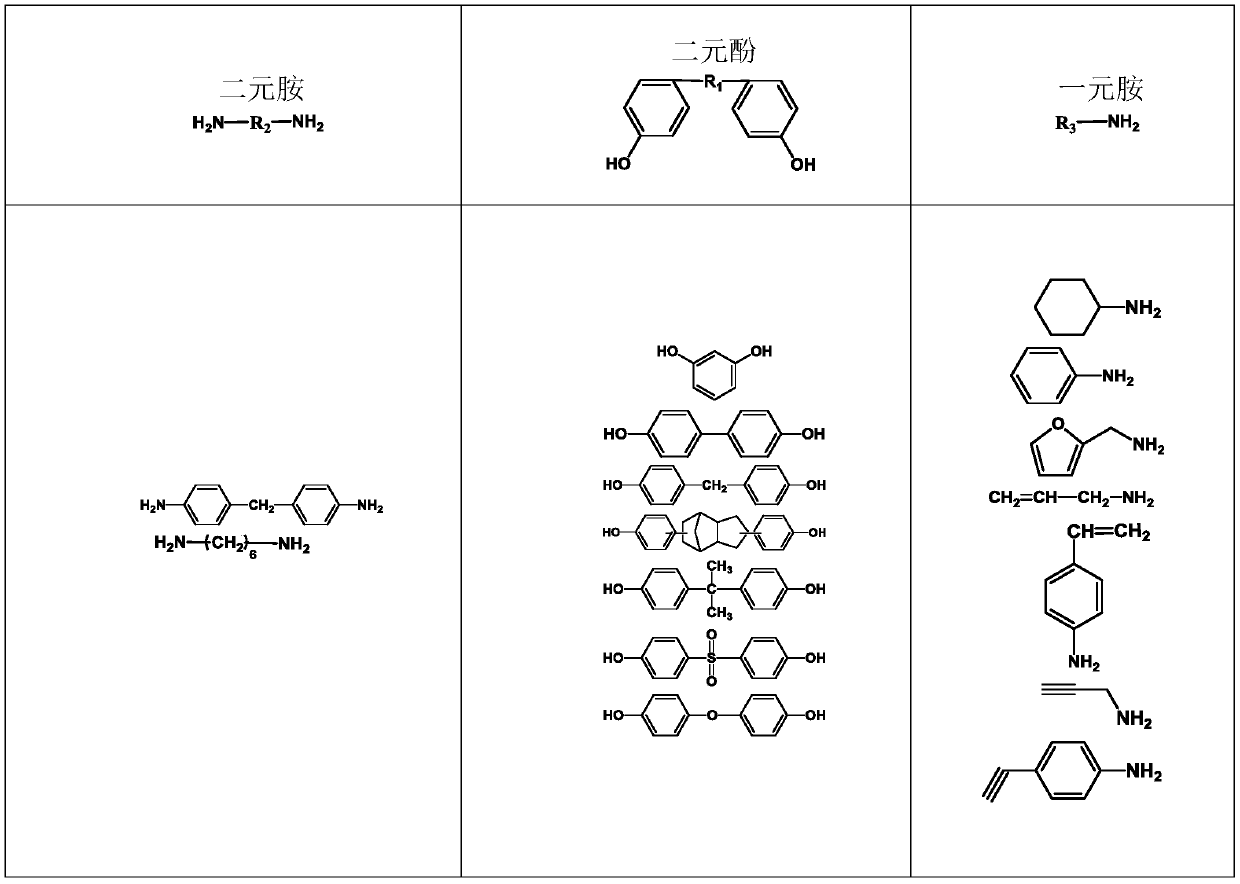
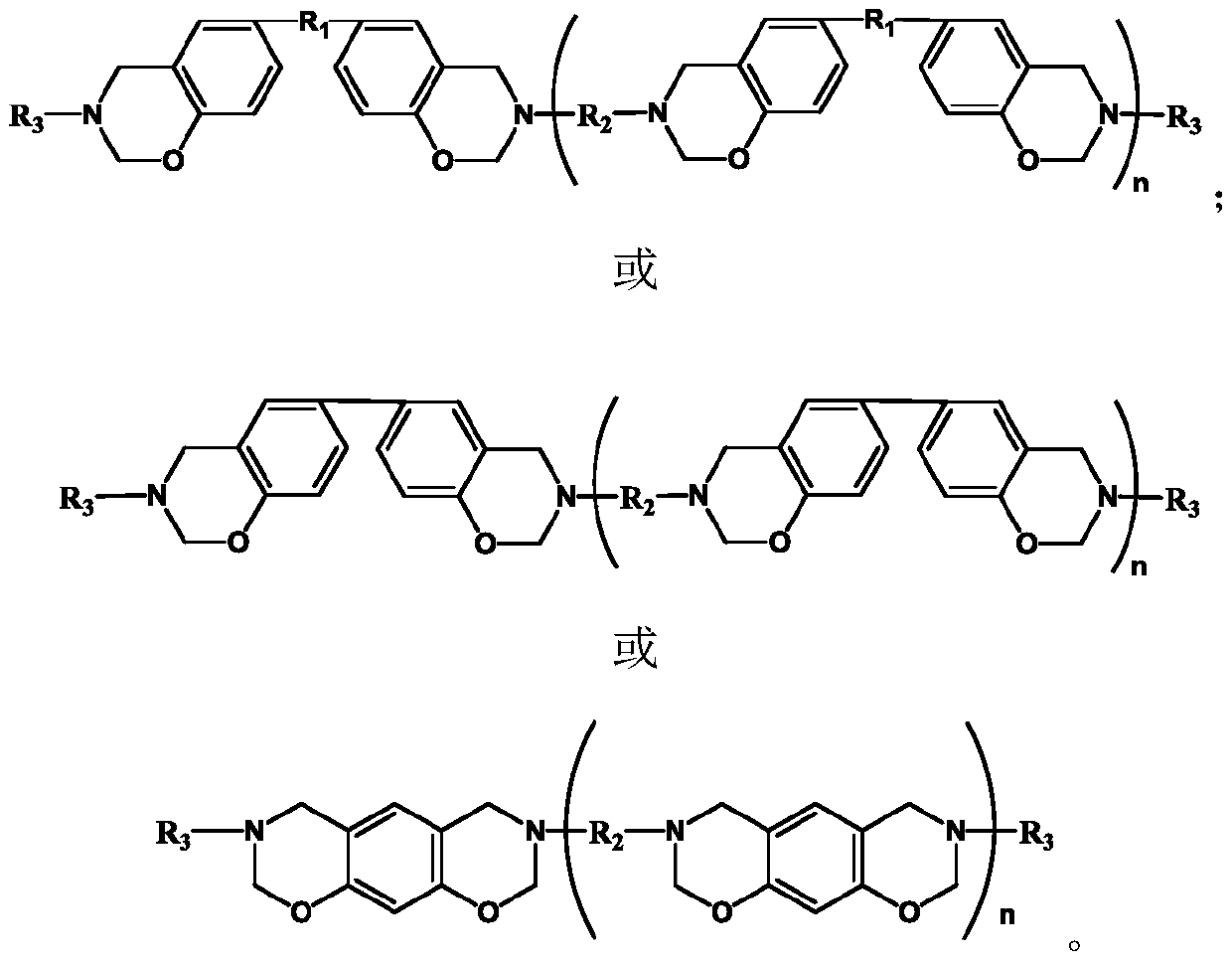
Monoamine-terminated ultrahigh-frequency low-dielectric main chain benzoxazine copolymer oligomer, copolymer resin and preparation method thereof
A benzoxazine copolymer resin, benzoxazine technology, applied in the field of organic polymer materials, can solve problems such as not being able to meet requirements well, and achieve the effects of excellent thermal properties, high crosslinking density, and improved dielectric properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]First add 0.04mol 1,6-hexamethylenediamine, 0.04mol bisphenol A, and 0.16mol paraformaldehyde into a 250mL three-necked flask equipped with a condenser, magnetic stirring, and a thermometer, then add 60mL of xylene, mix well and heat React at 120°C for 12 hours, then add 0.02mol aniline, 0.01mol bisphenol A, and 0.04mol paraformaldehyde and react for 12 hours, wherein the molar ratio of aldehyde groups, phenolic hydroxyl groups and amino functional groups in each added reactant is 2:1: The molar ratio of amino functional groups in 1,1,6-hexanediamine and aniline is 4:1. After the reaction, pour the reaction solution into 100mL methanol solution (concentration 40wt%) to obtain a suspension, let it stand for 24 hours, remove the supernatant to obtain a precipitate, vacuum dry the precipitate at 50°C for 8 hours, and finally grind the dried product The obtained powder is the main chain benzoxazine copolymer oligomer.
[0033] The molecular structural formulas of 1,6-hexame...
Embodiment 2
[0040] First add 0.04mol 1,6-hexamethylenediamine, 0.04mol bisphenol A, and 0.16mol paraformaldehyde into a 250mL three-necked flask equipped with a condenser, magnetic stirring, and a thermometer, then add 60mL of xylene, mix well and heat React at 120°C for 12 hours, then add 0.02mol p-ethynylaniline, 0.01mol bisphenol A, and 0.04mol paraformaldehyde and react for 12 hours, wherein the molar ratio of aldehyde groups, phenolic hydroxyl groups and amino functional groups in each added reactant is 2 :1:1, the molar ratio of amino functional groups in 1,6-hexanediamine and p-ethynylaniline is 4:1. After the reaction, pour the reaction solution into 100mL methanol solution (concentration 40wt%) to obtain a suspension, let it stand for 24 hours, remove the supernatant to obtain a precipitate, vacuum dry the precipitate at 50°C for 8 hours, and finally grind the dried product The obtained powder is the main chain benzoxazine copolymer oligomer.
[0041] The molecular structural fo...
Embodiment 3
[0048] Add 0.025mol 4,4'-diaminodiphenylmethane, 0.05mol p-vinylaniline, 0.05mol bisphenol A, and 0.2mol paraformaldehyde into a three-necked flask equipped with a condenser, magnetic stirring, and a thermometer, and add 51mL of toluene , heated to 100°C for 24 hours, the molar ratio of aldehyde group, phenolic hydroxyl group and amino functional group is 2:1:1, and the molar ratio of amine functional group in 4,4'-diaminodiphenylmethane and p-vinylaniline is 1:1 . After the reaction, pour the reaction solution into 100mL methanol solution (concentration 80wt%) to obtain a suspension, let it stand for 24 hours, remove the supernatant to obtain a precipitate, vacuum dry the precipitate at 60°C for 6 hours, and finally grind the dried product The obtained powder is the main chain benzoxazine copolymer oligomer.
[0049] The molecular structural formulas of 4,4'-diaminodiphenylmethane, bisphenol A and p-vinylaniline used in this example are respectively:
[0050]
[0051] mo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com