Thermally activated delayed fluorescence luminescent material containing nitrogen hetero helicene parent nucleus and applications of thermally activated delayed fluorescence luminescent material in electroluminescent devices
An electroluminescent device, a technology of thermal activation delay, applied in the direction of luminescent materials, electric solid devices, electrical components, etc., to achieve the effects of flexible derivation methods, simple synthesis, and high color purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
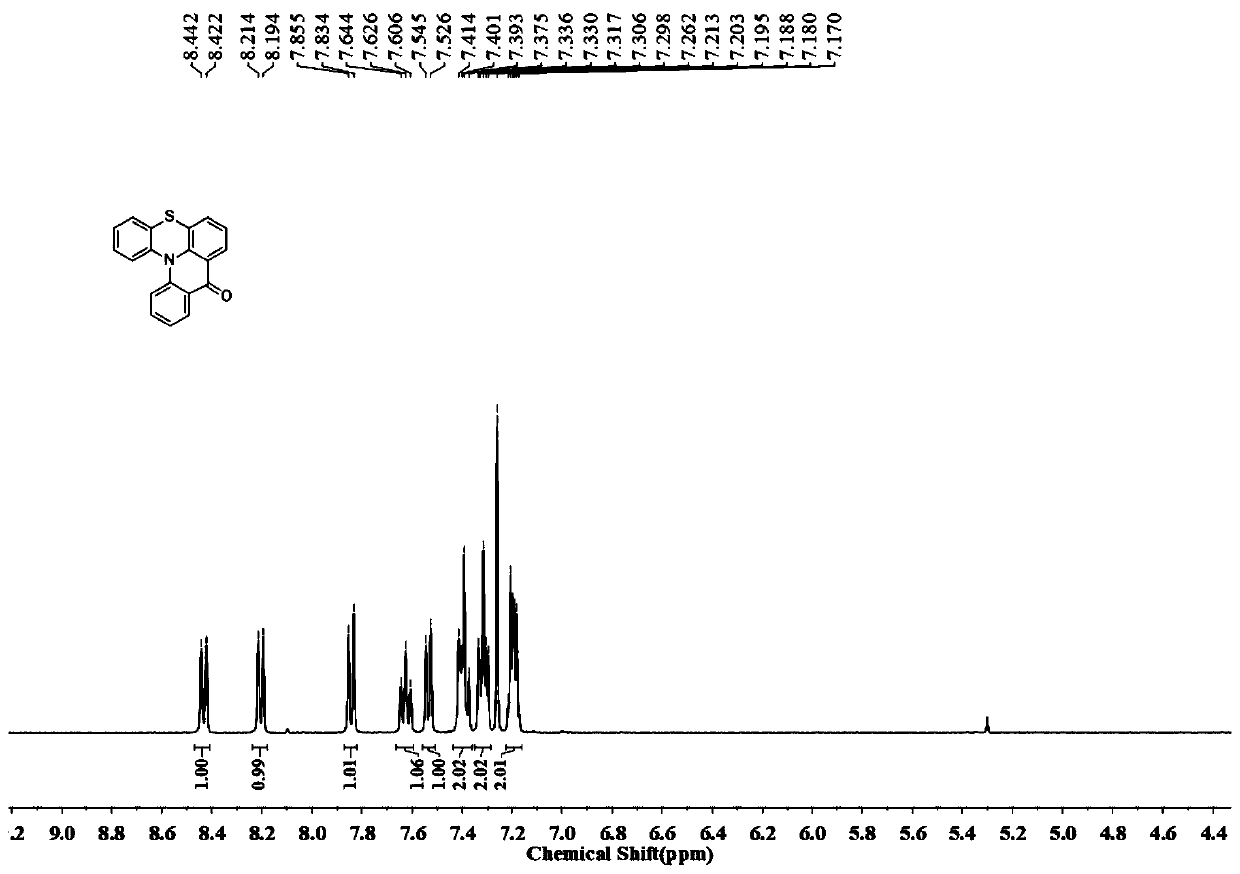
[0043] Among the w-1 compounds shown in the present invention, when R 1 =R 2 =R 3 When =H, it is compound 3 of the following formula; in w-2 compound, when R 1 =R 2 =R 3 When =H, it is compound 4 of the following formula. The preparation of compound 3 and 4 can be synthesized according to following reaction, and its synthetic route is as follows:
[0044]
[0045] Preparation of compound 1
[0046] Phenothiazine (1.75 g, 8.78 mmol), methyl 2-iodobenzoate (2.3 g, 8.78 mmol), cuprous iodide (167 mg, 0.88 mmol) and potassium carbonate (1.21 g, 8.78 mmol) were added to di In chlorobenzene (30mL), react at 190°C for 24h under the protection of nitrogen, cool, filter, wash the solid with dichloromethane, concentrate the filtrate, and perform silica gel column chromatography (petroleum ether:dichloromethane=2:1) to obtain 2.44g of white solid , yield 83.2%.
[0047] MS(EI):m / z 333.1[M + ]. Elemental analysis, theory:: C, 72.05; H, 4.54; N, 4.20; O, 9.60; S, 9.62; actua...
Embodiment 2
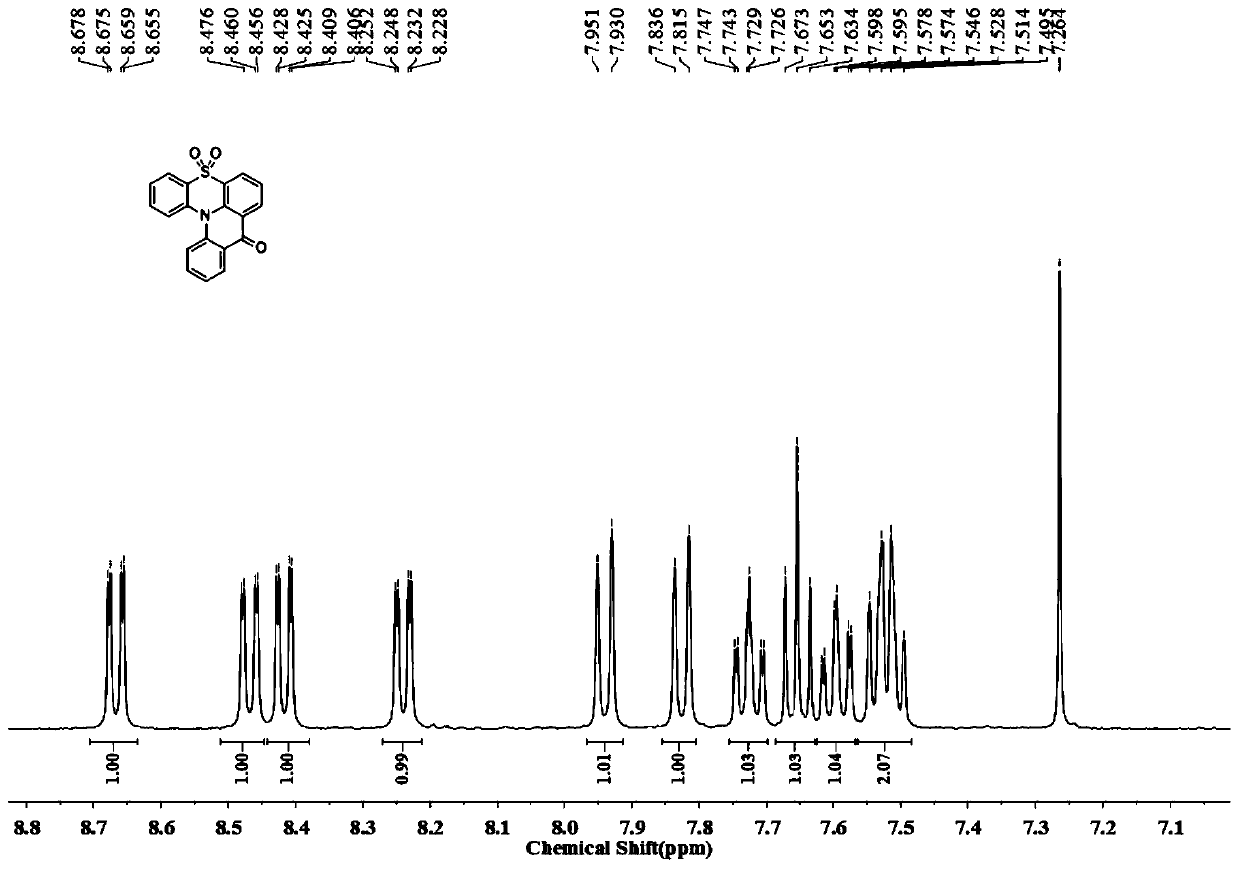
[0057] In w-6, when R 1 =R 2 =H, , it is compound 9 of the following formula; when R 1 =R 2 =H, , it is compound 10 of the following formula.
[0058] The preparation method of compound 9, 10 can be prepared according to the following reaction formula and its synthetic route is as follows:
[0059]
[0060] Compounds 5-8 were operated as in Example 1.
[0061] Preparation of compound 9
[0062] Add compound 8 (0.41g, 1mmol), 10H-phenothiazine (0.2g, 1mmol), tris(dibenzylideneacetone) dipalladium (92mg, 0.1mmol), potassium carbonate (0.28 g, 2mol) and tri-tert-butylphosphine (10% toluene solution, 1.4mL), under nitrogen protection, heated to reflux and stirred for 24h. Cool to room temperature, filter, wash the filter residue with dichloromethane, collect the filtrate, distill off the solvent under reduced pressure, and the residue is an eluent with a mixed solvent of petroleum ether (PE) and dichloromethane (DCM) (PE:DCM=5: 1, V:V), separated by column chromatogr...
Embodiment 3
[0066] In the shown w-3 core compound provided by the present invention, when R 1 =R 2 =R 3 When =H, it is compound 13 in the following formula. In the shown w-4 core compound provided by the present invention, when R 1 =R 2 =R 3 When =H, it is compound 14 in the following formula. Compounds 13 and 14 can be prepared according to the following reaction formula. :
[0067]
[0068] Preparation of Compound 11
[0069] Phenothiazine (13.15g, 0.066mol), diethyl 2,5-dibromoterephthalate (11.4g, 0.03mol), cuprous iodide (570 mg, 3mmol) and potassium carbonate (9.11g, 0.0668mol) was added to dichlorobenzene (150mL), reacted at 190°C under nitrogen protection for 24h, cooled, filtered, washed the solid with dichloromethane, and the filtrate was concentrated and then chromatographed on a silica gel column (petroleum ether: dichloromethane = 2:1 ) to obtain brown solid 11.34g, yield 61.3%.
[0070] MS(EI):m / z 616.1[M + ]. Elemental Analysis, Theory: C, 70.11; H, 4.58; N, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com