Tumor-targeted GRP analogue and application thereof
A technology of tumor targeting and analogues, applied in the field of medical bioengineering, can solve different problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
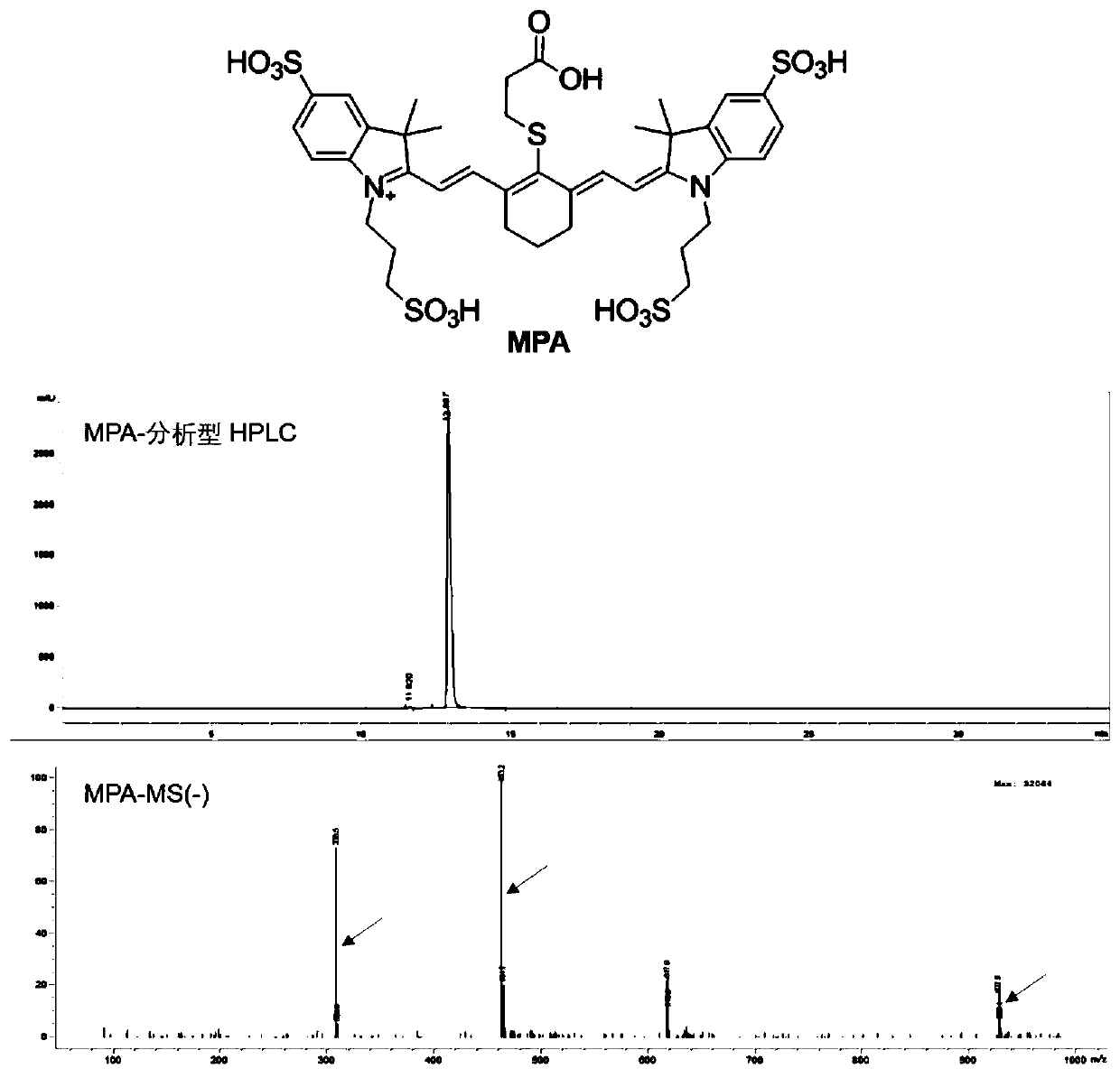
[0071] The synthesis of embodiment 1 near-infrared fluorescent dye MPA
[0072] For the synthesis steps, refer to an invention patent previously applied by our research group, authorized patent number: CN101440282, the main synthesis steps are as follows:
[0073] 1. Add 30mL of glacial acetic acid to a mixture of p-hydrazinobenzenesulfonic acid (6g), methyl isopropyl ketone (7mL) and sodium acetate (2g). The obtained brown suspension was heated to boiling and refluxed for 16 hours. Then filter while hot with a sintered glass filter to remove unreacted suspended matter. After the filtrate was cooled to room temperature, the product was precipitated with dichloromethane, and the product was brown (2,2,3-trimethyl[3H]-indole-5-sulfonic acid).
[0074] 2. Take 50 mL o-dichlorobenzene and add to 2,2,3-trimethyl[3H]-indole-5-sulfonic acid (15 g) and 1,3-propane sultone (4 mL). The mixture was heated to 130°C and held for 15 hours. The resulting solid was triturated with dichlor...
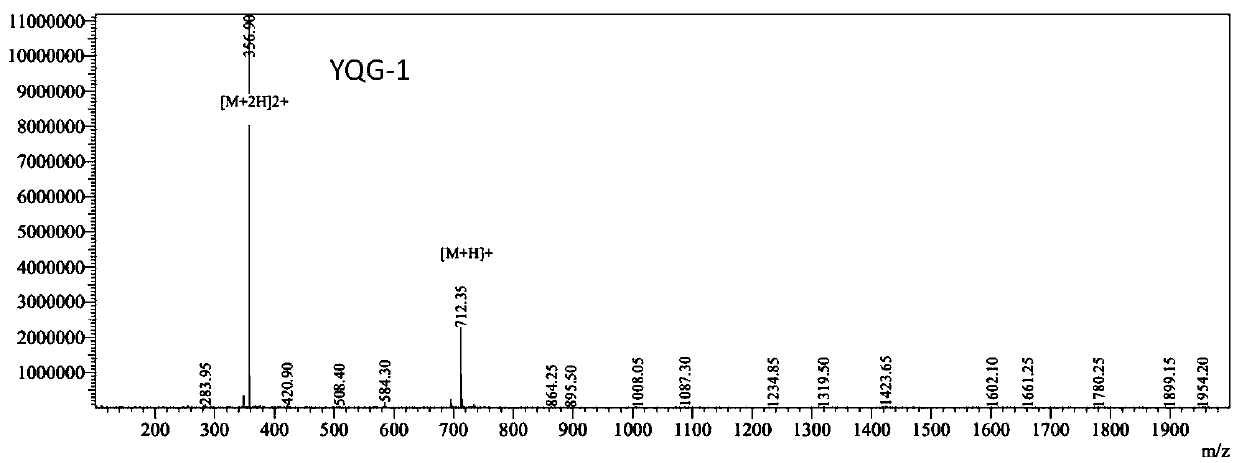
Embodiment 2Y
[0077] The synthesis of embodiment 2YQG-1
[0078] 1) Resin swelling
[0079] Weigh the Rink Amide MBHA resin, put it into the reaction column, add an appropriate amount of dichloromethane (DCM), blow nitrogen slightly for 10-30 minutes, and fully expand the resin. Remove the DCM solution, wash 3 times with DMF, and drain.
[0080] 2) Remove Fmoc
[0081] Add 20% hexahydropyridine DMF solution to the reaction column, and deprotect once every 5 minutes, once every 8 minutes. After the reaction was finished, it was washed 6 times with DMF.
[0082] 3) Coupling
[0083] Accurately weigh Fmoc-His(trt)-OH and O-benzotriazole-N,N,N',N'-tetramethyluronium tetrafluoroborate (TBTU) which is 3 times the mole number of the feed resin, Completely dissolve in DMF, add N,N-diisopropylethylamine (DIPEA) to activate the carboxyl group, then add the solution to the reaction column for reaction, detect after 1h, and the detection structure is positive. The Fmoc was decoupled sequentially ...
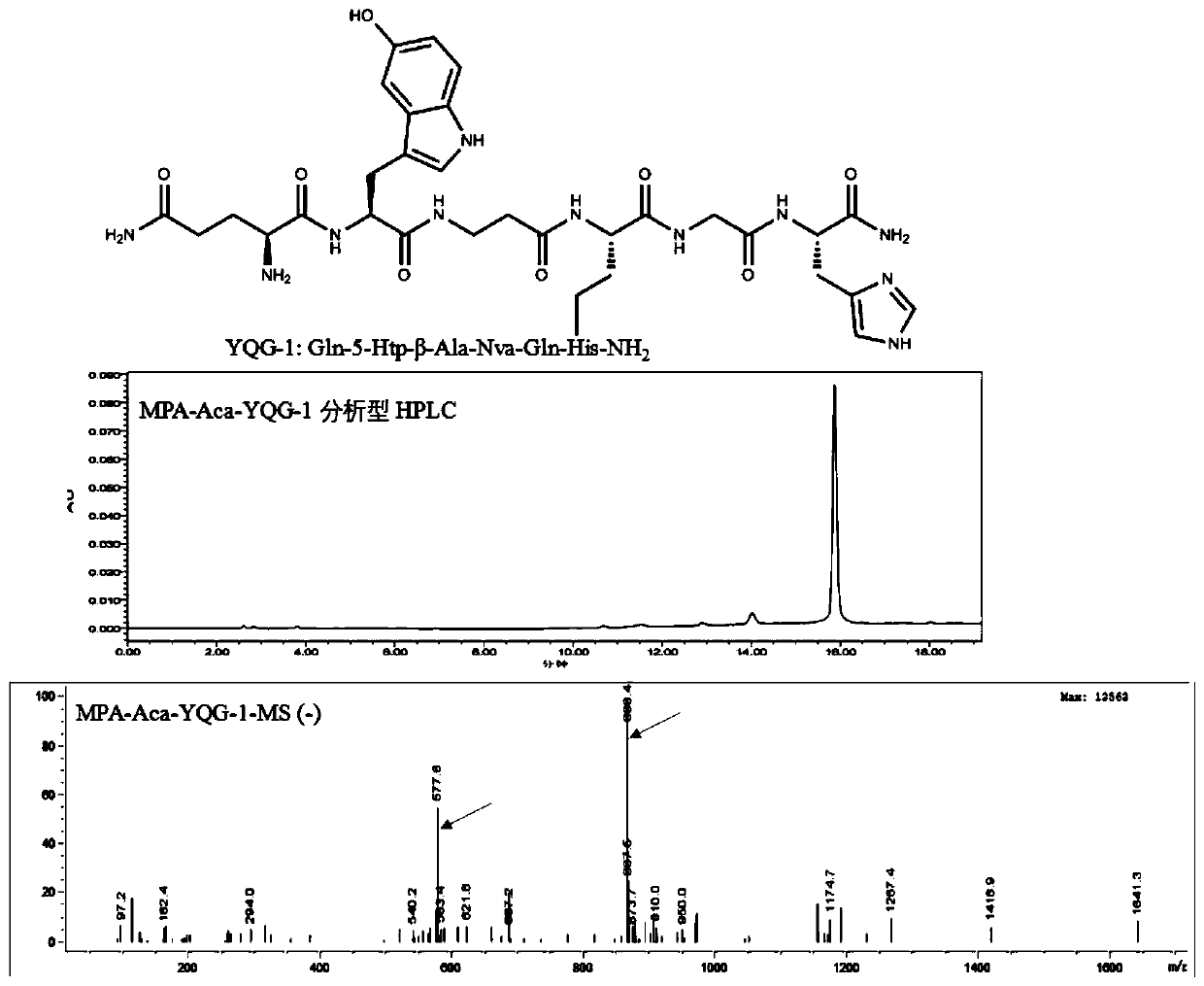
Embodiment 3
[0089] Example 3 Preparation of fluorescent targeting compound MPA-Aca-YQG-1
[0090] Weigh 10 mg of the Aca-YQG-1 compound synthesized by solid phase, and add 12.38 mg of the pure dye MPA to 200 μL of dimethyl sulfoxide (DMSO), and then add 2.3 mg of 1-(3-dimethylaminopropyl) -3-Ethylcarbodiimide hydrochloride (EDCI) coupling agent and 3.82mg N-hydroxysuccinimide (NHS), after mixing, add 4.1mg N,N-diisopropylethylamine (DIPEA), react overnight at room temperature, after the reaction is completed, use the preparative liquid phase to separate and purify, and the preparative liquid phase conditions are as follows: Agilent ZORBAX SB-C18 semi-preparative column (9.4 × 250mm, 5um ), gradient elution for 60 minutes, flow rate 2mL / min, wherein mobile phase A is ultrapure water (0.01% TFA), and B is acetonitrile (0.01% TFA). The elution gradient was set as: 95% A and 5% B at 0-5 minutes, 80% A and 20% B at 15 minutes, 50% A and 50% B at 45 minutes, 5% A and 95 at 60 minutes %B. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com