Polypeptide, application thereof and inhibitors or drugs or health products
A technology of health products and inhibitors, which is applied in the field of peptides and their applications and inhibitors or drugs or health products, which can solve the problems of changing treatment plans and not being able to cure Alzheimer's disease, so as to improve memory and have good application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] The preparation of embodiment 1 polypeptide Phe-Pro-Ser-Ile-Val-Gly-Arg-Pro
[0015] A method combining LC-MS / MS with Shotgun proteomics technology was used. Using sika deer antler as raw material, through enzymatic hydrolysis, centrifugation, purification and LC-MS / MS analysis, combined with the characteristics of structure-activity relationship, the peptides with inhibitory effects on DPP-Ⅳ and PEP were screened.
[0016] The specific method is as follows:
[0017] Freeze-dry fresh sika antler and crush it, add deionized water to make the antler concentration 33.3g / L, stir well, add trypsin with 0.5% antler mass (W / W), and enzymolyze at 40°C for 3 hours; enzymolysis At the end, pass the enzymatic solution through an 80-mesh sieve, and extract the residue once in the same way. Heat the two enzymatic hydrolysis solutions to 90°C for 15 minutes, filter through 8 layers of gauze and 200-mesh sieve successively, centrifuge the obtained filtrate at a speed of 10000g for 1...
Embodiment 2
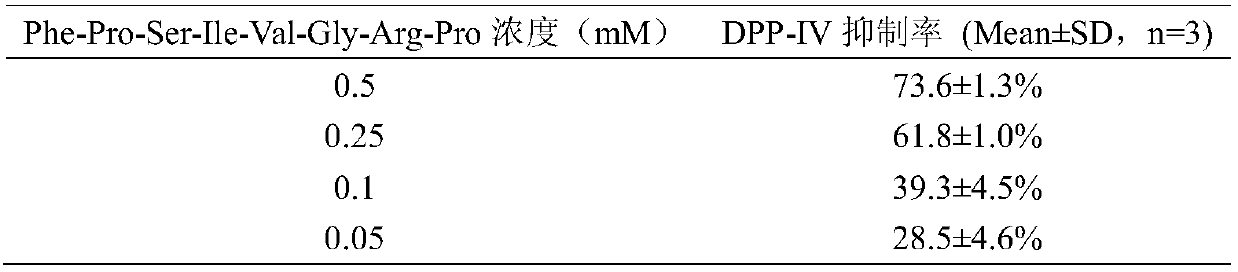
[0021] Example 2 Detection of DPP-IV inhibitory activity of polypeptide Phe-Pro-Ser-Ile-Val-Gly-Arg-Pro
[0022] principle
[0023]For peptides with X-Pro and X-Ala at the N-terminal, DPP-IV can selectively excise the dipeptide, and GLP-1 has an X-Ala structure, so DPP-IV can cause more than 95% of GLP-1 to occur degradation. In this method, Gly-Pro-p-nitroanilide is used instead of GLP-1 as the simulated substrate of DPP-Ⅳ. After DPP-Ⅳ cuts off Gly-Pro, the generated p-nitroaniline has a characteristic absorption peak at 405nm, which can be calculated Inhibitory activity of DPP-IV.
[0024] experimental method:
[0025] The polypeptide Phe-Pro-Ser-Ile-Val-Gly-Arg-Pro used in the experiment was synthesized by Nanjing Jiepei Biotechnology Co., Ltd. with a purity of >95%. Dissolve the polypeptide Phe-Pro-Ser-Ile-Val-Gly-Arg-Pro in 100 mM Tris-HCl buffer (pH=8.0), add 25 μL of Phe-Pro-Ser-Ile-Val-Gly to a 96-well plate -Arg-Pro solution and 25μL 1.59mM Gly-pro-p-nitroanilide...
Embodiment 3
[0032] Example 3 Detection of PEP inhibitory activity of polypeptide Phe-Pro-Ser-Ile-Val-Gly-Arg-Pro
[0033] 1. Principle
[0034] PEP can specifically hydrolyze small molecular weight polypeptides at the carboxyl terminus of proline. Therefore, this method uses Z-Gly-Pro-4-nitroanilide as the substrate of PEP. After Z-Gly-Pro-4-nitroanilide is cut off by PEP to cut Z-Gly-Pro, yellow p-nitroanilide is produced, which has a characteristic at 405nm. Absorption peak, according to the change of absorbance at 405nm before and after adding the sample and substrate, the inhibitory activity of the sample for PEP can be calculated.
[0035] 2. Method
[0036] (1) Polypeptide Phe-Pro-Ser-Ile-Val-Gly-Arg-Pro solution: The polypeptide Phe-Pro-Ser-Ile-Val-Gly-Arg-Pro used in the experiment was synthesized by Nanjing Jiepei Biotechnology Co., Ltd., the purity >95%. Phe-Pro-Ser-Ile-Val-Gly-Arg-Pro was dissolved in 10 mM PBS buffer (10 mM PBS, 137 mM NaCl and 2.7 mM KCl, pH 7.0).
[003...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com