Detection method for content of enantiomer in L-alanine isopropyl ester
A technology of isopropyl alanine and enantiomers, which is applied in the field of high performance liquid chromatography detection of enantiomer content, can solve the enantiomerization of L-isopropyl alanine hydrochloride No pharmacopoeia and other problems, to achieve the effect of strong specificity, high sensitivity, good accuracy and durability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
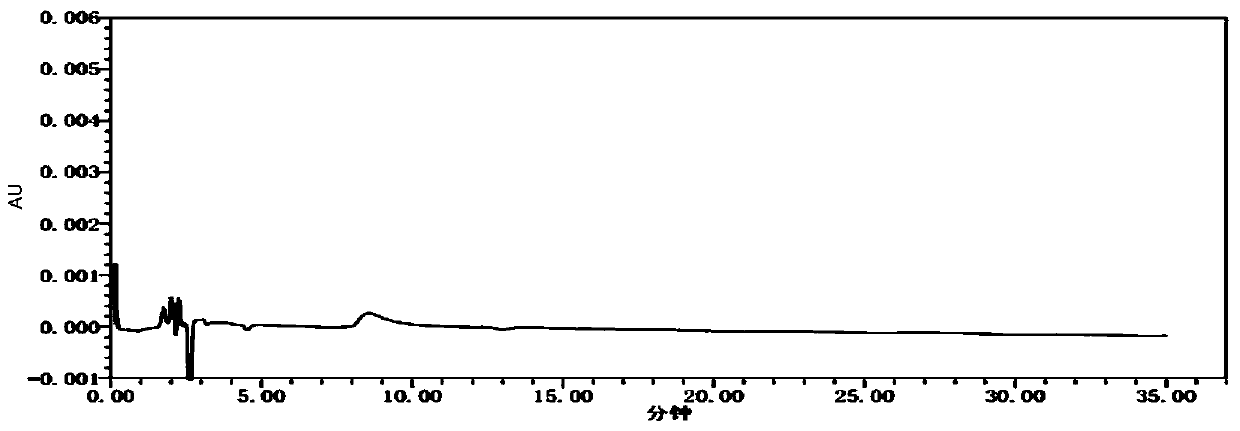
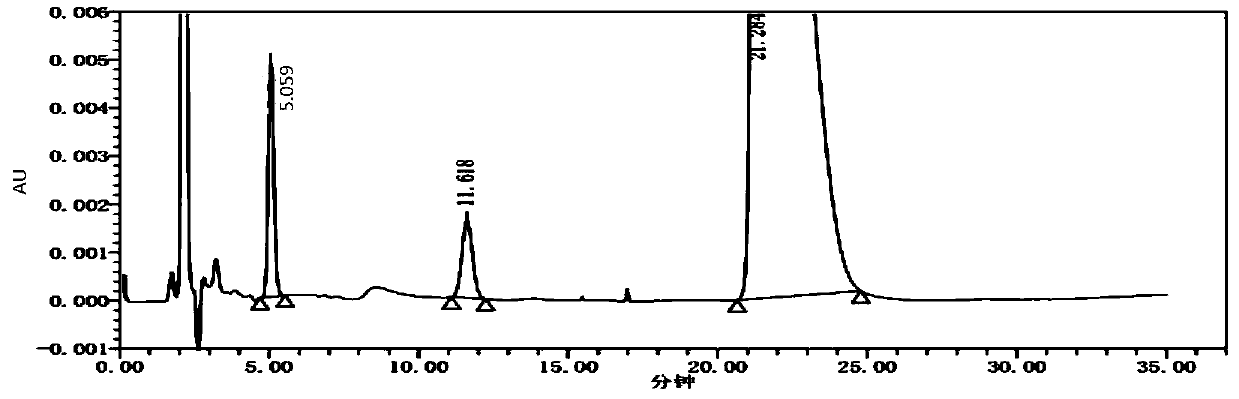
[0028] specificity test
[0029] Adopt the method among the present invention.
[0030] Chromatographic column: CROWNPAKCR (+) (4.0mm×150mm, 5μm)
[0031] Mobile phase: Perchloric acid aqueous solution at pH 1.0
[0032] Flow rate: 0.5ml / min
[0033] Column temperature: 20°C
[0034] Detection wavelength: 205nm
[0035] Injection volume: 20μl
[0036] Experimental steps:
[0037] Blank solvent: purified water
[0038] Preparation of system suitability solution: Take the appropriate amount of impurity TN-SM2-R2 reference substance, enantiomer reference substance and L-alanine isopropyl ester hydrochloride reference substance respectively, add purified water to dissolve and dilute to make each A mixed solution containing about 50 μg of impurity TN-SM2-R2 and 50 μg of enantiomers in 1 ml, and 2.5 mg of L-alanine isopropyl ester hydrochloride is used as a system suitability solution.
[0039] Preparation of sample solution: Take an appropriate amount of this product, weigh...
Embodiment 2
[0048] Limit of detection and limit of quantitation tests.
[0049] The chromatographic method in Example 1 was used.
[0050] Experimental steps:
[0051] Blank solvent: purified water
[0052] Limit of detection and limit of quantitation solution: Take appropriate amount of L-alanine isopropyl ester hydrochloride and enantiomer reference substance, add anhydrous purified water to prepare solutions with a series of concentrations, and measure according to the law. The quantification limit and detection limit of the substance were judged by the signal-to-noise ratio.
[0053] The results are shown in Table 1 below.
[0054]
Embodiment 3
[0056] Linearity and Range
[0057] The chromatographic method in Example 1 was used.
[0058] Experimental steps:
[0059] Blank solvent: purified water
[0060] Linear solution: Take an appropriate amount of L-alanine isopropyl ester hydrochloride and enantiomer reference substance, add purified water to prepare solutions with a series of concentrations, and measure according to the law.
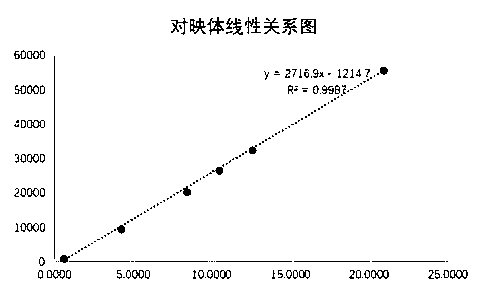
[0061] Results: The enantiomer of L-alanine isopropyl ester hydrochloride had a good linear relationship in the concentration range of 0.5214 μg / ml-20.8560 μg / ml, and the concentration of L-alanine isopropyl ester hydrochloride was 0.5410 μg / ml The linear relationship is good in the concentration range of ml-21.6560μg / ml. The specific results are shown in Table 2 below, and the linear relationship diagram is shown in the appendix Figure 5 , Figure 6 .
[0062]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com