Electrolyte preparation method for inhibiting self-discharge of solid lithium battery
A solid electrolyte and self-discharge technology, which is applied in electrolytes, secondary batteries, circuits, etc., can solve the problem that the self-discharge of solid-state lithium batteries is difficult to effectively control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
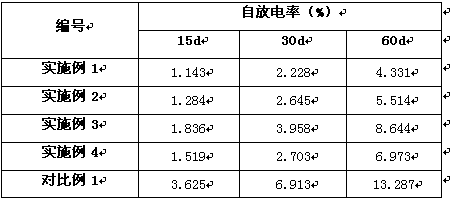
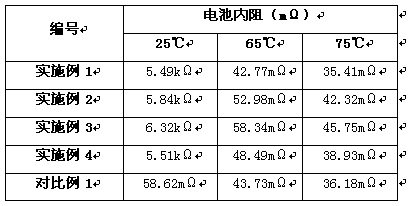
Embodiment 1
[0034] (a). Take V respectively 2 o 5 Powder, carbon black, and tungsten oxide powder were passed through a 300-mesh sieve, and the under-sieve was taken, and 270g of V 2 o 5 Mix the powder with 8g of carbon black and 10g of tungsten oxide powder under the condition of ammonia gas, and carry out dry ball milling in a ball mill, and the time of dry ball milling is 20 minutes;
[0035] (b). Place the powder after dry ball milling in step (a) under the protection of nitrogen for heat reduction, and the heat reduction temperature is 750°C to obtain V 0.95 W 0.05 o 2 powder;
[0036] (c). The V described in step (b) 0.95 W 0.05 o 2 Powder 100g is mixed with 10g sodium carboxymethylcellulose and pressed into a film of 0.25mm, Li 2 O, La 2 o 3 , ZrO 2 According to the molar ratio of Li:La:Zr=7.7:3:2, 500g of powder mixed with 50g of sodium carboxymethylcellulose was mixed and pressed into a 2mm film, and the two films were laminated and compacted into a 2.5mm sheet .
...
Embodiment 2
[0040] (a). Take V respectively 2 o 5 Powder, carbon black, and tungsten oxide powder were passed through a 300-mesh sieve, and the under-sieve was taken, and 270g of V 2 o 5 Mix the powder with 10g of carbon black and 3.5g of tungsten oxide powder under the condition of ammonia gas, and carry out dry ball milling in a ball mill, and the time of dry ball milling is 20 minutes;
[0041] (b). Place the powder after dry ball milling in step (a) under the protection of nitrogen for heat reduction, and the heat reduction temperature is 750°C to obtain V 0.98 W 0.02 o 2 powder;
[0042] (c). The V described in step (b) 0.98 W 0.02 o 2 Powder 75g is mixed with 7.5g sodium carboxymethylcellulose and pressed into a film of 0.3mm, Li 2 O, La 2 o 3 , ZrO 2 According to the molar ratio of Li:La:Zr=7.7:3:2, 500g of powder mixed with 50g of sodium carboxymethyl cellulose was mixed and pressed into a 2.3mm film, and the two films were laminated and compacted into a 2.9mm film. F...
Embodiment 3
[0046] (a). Take V respectively 2 o 5 Powder, carbon black, and tungsten oxide powder were passed through a 300-mesh sieve, and the under-sieve was taken, and 250g of V 2 o 5 Mix the powder with 10g of carbon black and 6.5g of tungsten oxide powder under the condition of ammonia gas, and carry out dry ball milling in a ball mill, and the time of dry ball milling is 20 minutes;
[0047] (b). Place the powder after dry ball milling in step (a) under the protection of nitrogen for heat reduction, and the heat reduction temperature is 750°C to obtain V 0.97 W 0.03 o 2 powder;
[0048] (c). The V described in step (b) 0.97 W 0.03 o 2 Powder 100g is mixed with 10g sodium carboxymethylcellulose and pressed into a film of 0.4mm, Li 2 O, La 2 o 3 , ZrO 2 According to the molar ratio of Li:La:Zr=7.7:3:2, 500g of powder mixed with 50g of sodium carboxymethylcellulose was mixed and pressed into a 2.5mm film, and the two films were laminated and compacted into a 3.3mm film. Fl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com