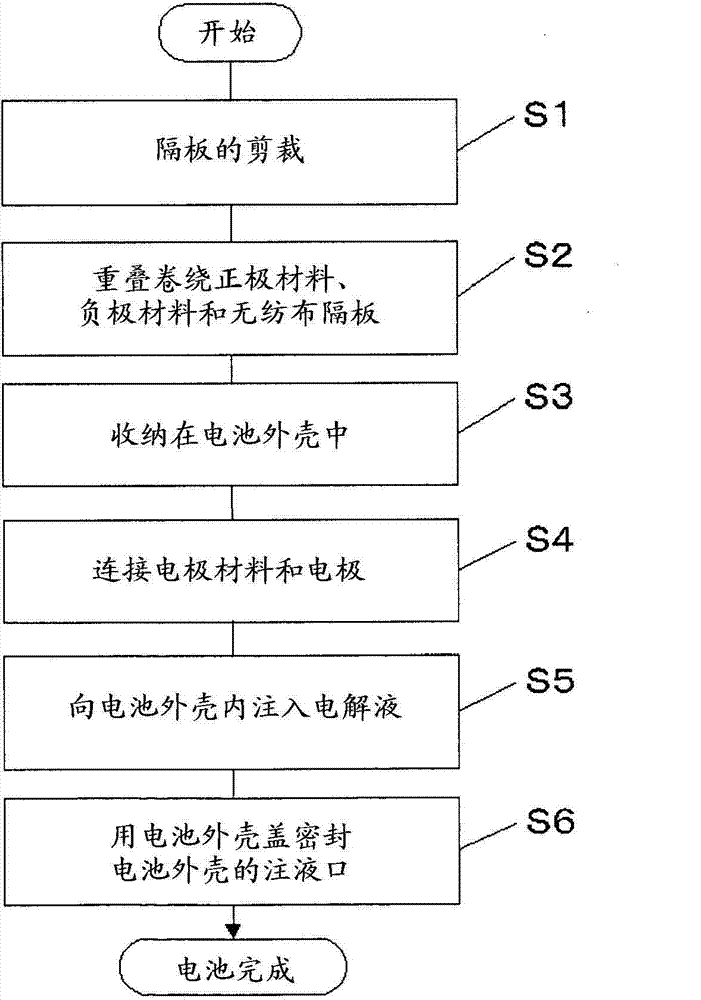
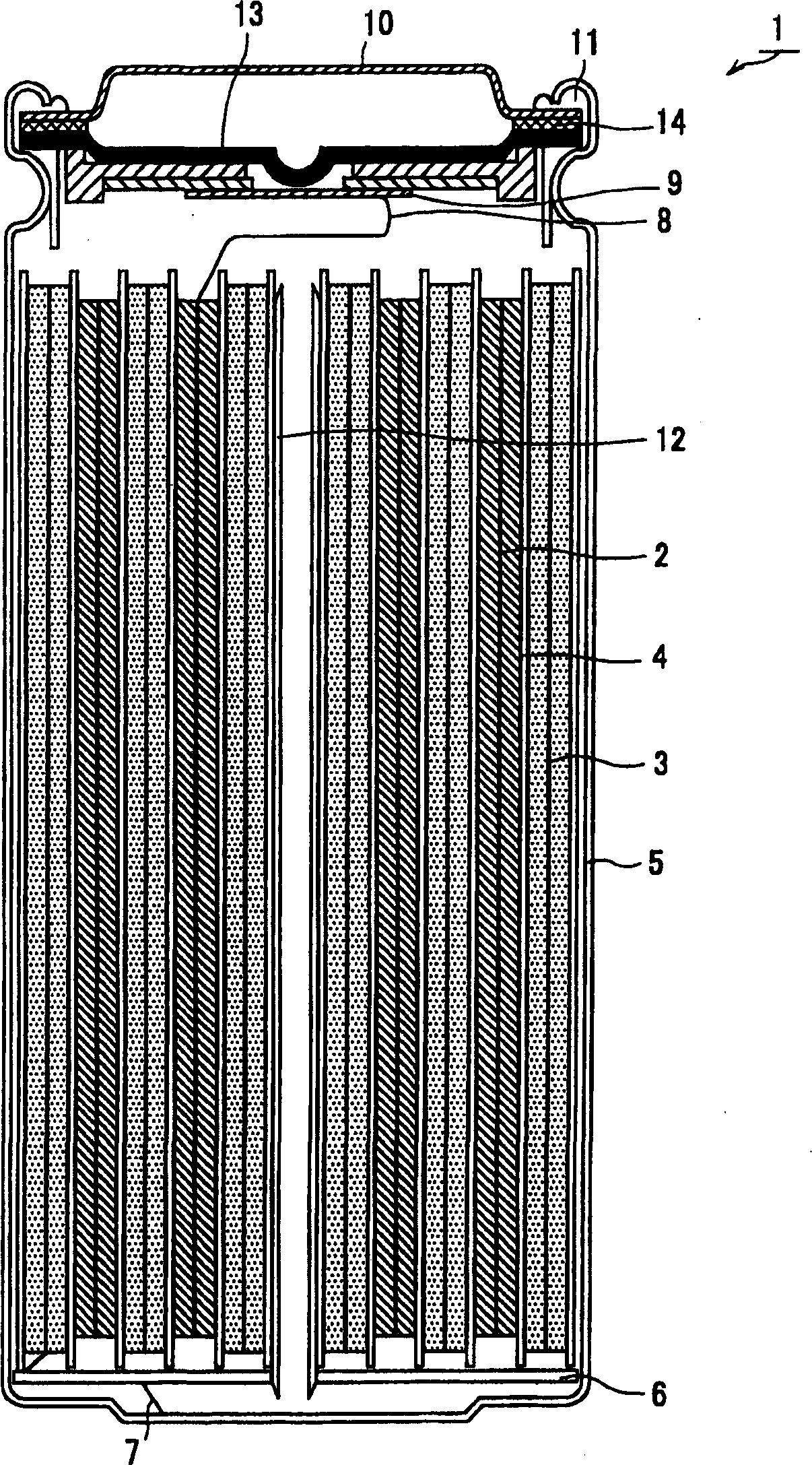
Patents
Literature
42results about How to "Inhibition of self-discharge" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
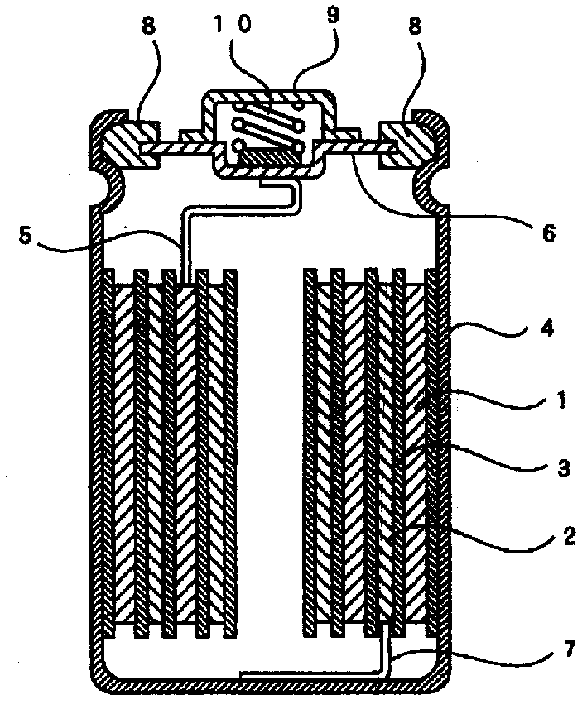
Negative electrode mixture or gel electrolyte, and battery using said negative electrode mixture or said gel electrolyte
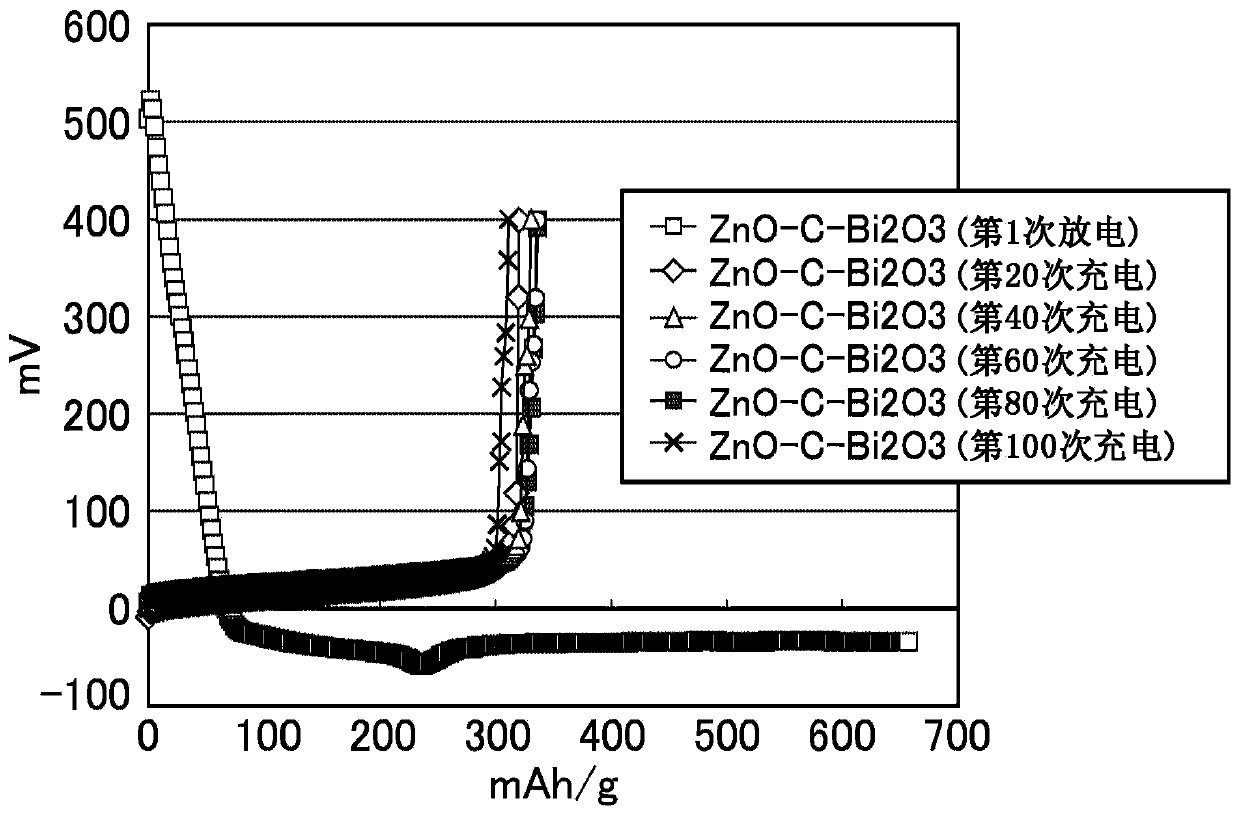
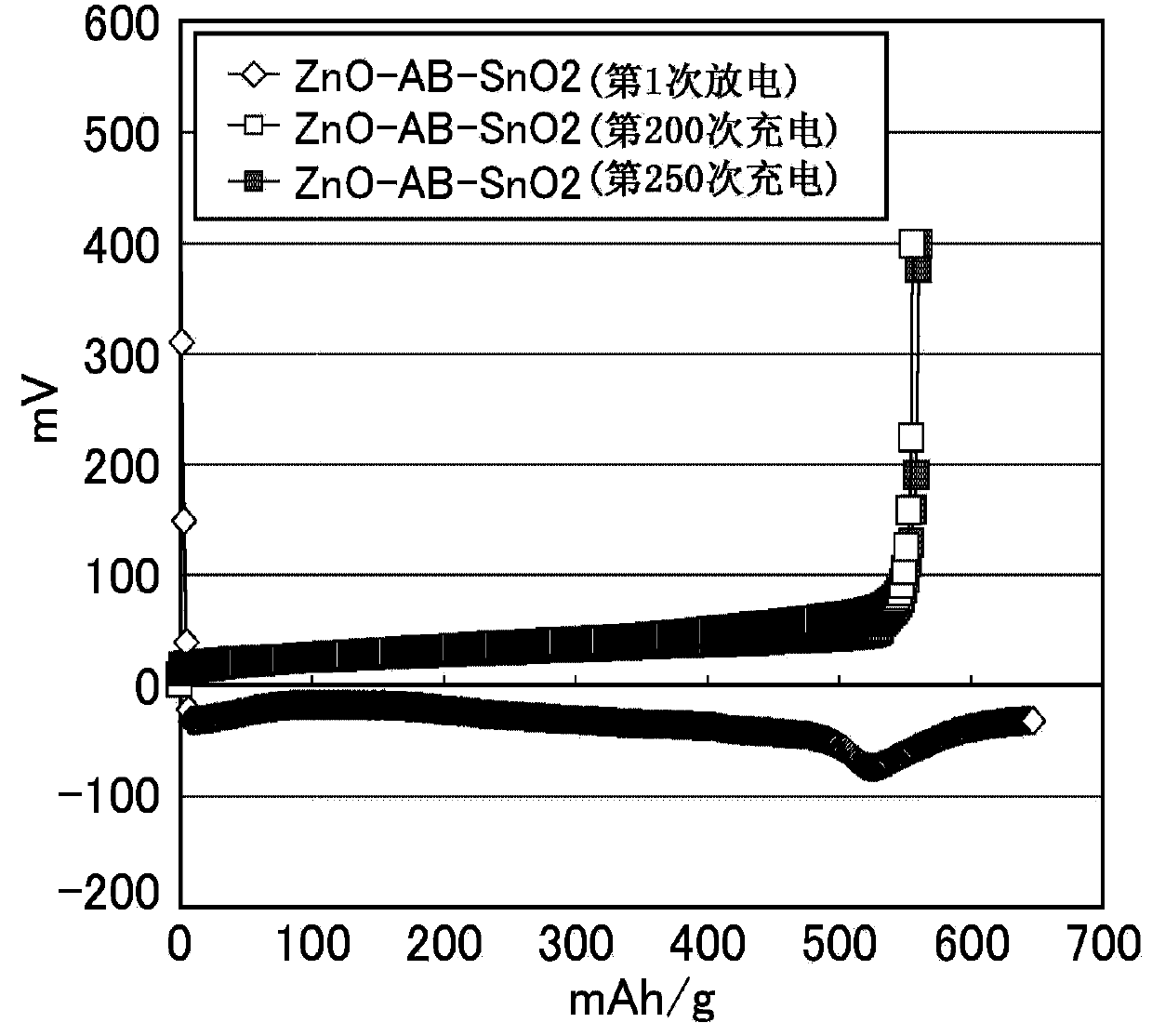
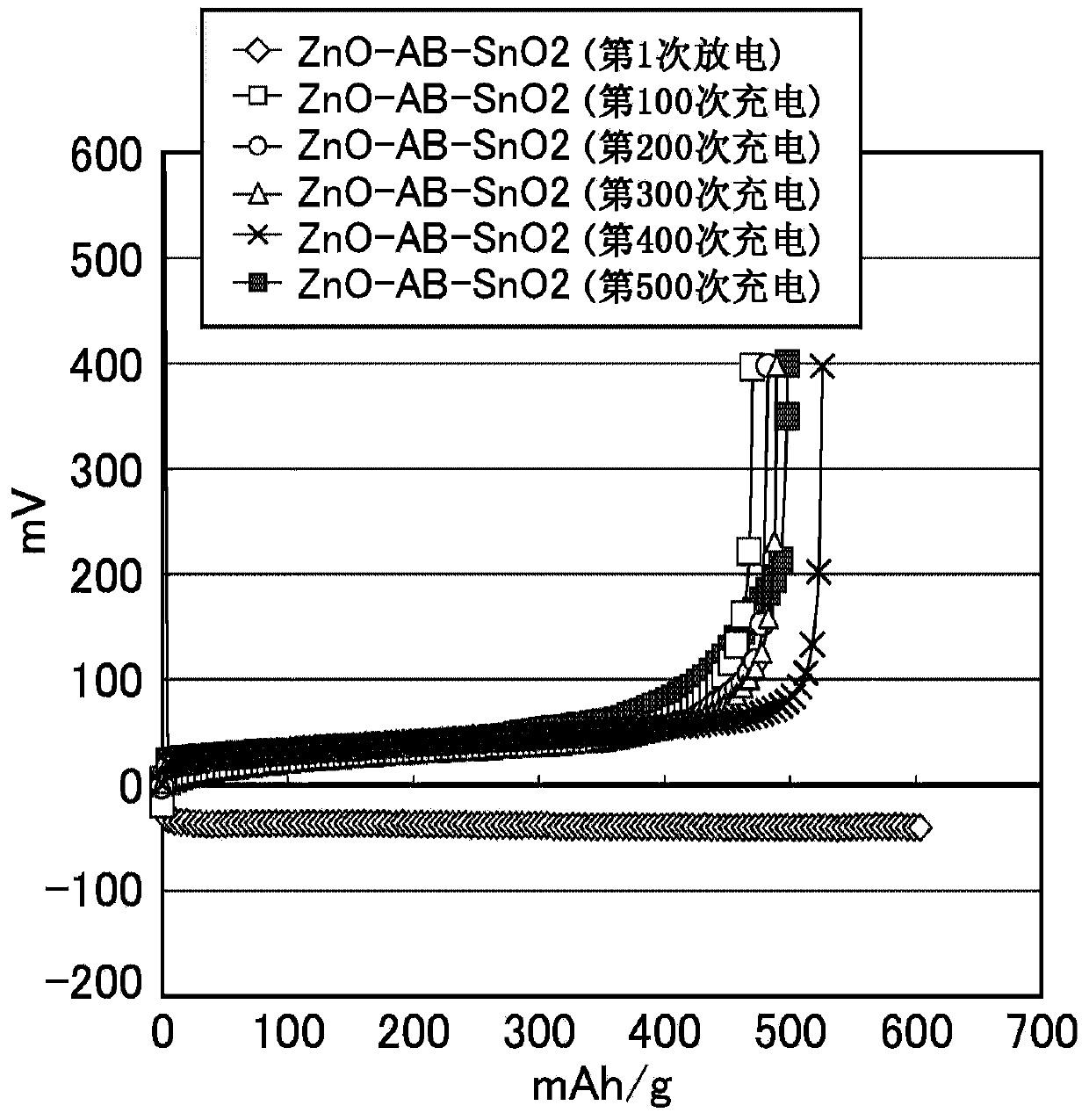
ActiveCN103748710AExcellent cycle characteristicsHigh rate characteristicsFuel and primary cellsFuel and secondary cellsCross-linkShape change
The purpose of the present invention is to provide a zinc negative electrode mixture for forming the negative electrode for a safe and economic battery exhibiting excellent battery performance, and a gel electrolyte or a negative electrode mixture which can be suitably used for forming a storage battery exhibiting excellent battery performances such as a high cycle characteristics, rate characteristics, and coulombic efficiency while inhibiting passivation and morphology of an electrode active material such as dendrite and shape change in the electrode active material. Another purpose of the present invention is to provide a battery using the zinc negative electrode mixture or the gel electrolyte. A zinc negative electrode mixture (1) containing a zinc-containing compound and a conductive auxiliary agent, wherein the zinc-containing compound and / or the conductive auxiliary agent contain particles having an average particle size of 1000 [mu]m or less and / or particles having an aspect ratio (vertical / lateral) of 1.1 or more. A gel electrolyte (2) used in a battery, the gel electrolyte being characterized by having a cross-linking structure formed by a multivalent ion and / or an inorganic compound. A negative electrode mixture (3) used in a battery, the negative electrode mixture being characterized by containing a negative electrode active material and a polymer.
Owner:NIPPON SHOKUBAI CO LTD
Power storage device separator
InactiveCN102177561AAvoid short circuitIncreased durabilityHybrid capacitor separatorsElectrolytic capacitorsElectrical resistance and conductanceOrganic solvent
Provided is a power storage device separator that is realized in the form of a heat-resistant, solvent-resistant, and dimensionally stable thin film. Also provided is a power storage device separator that can be realized in the form of a thin film and that has excellent ion permeability and low resistance. Shorting between electrodes and self-discharge are unlikely to occur in the latter separator, and in addition, durability is excellent even after long periods of use in high-temperature environments in the presence of organic solvents and ionic solutions.
Owner:TOMOEGAWA PAPER CO LTD
Lead-free and mercury-free alkali button battery zinc cream and preparation method thereof
ActiveCN101901894AWon't happenWith explosion-proof functionPrimary cell electrodesState of artPowder mixture
The invention relates to the technical field of battery manufacture, in particular to lead-free and mercury-free alkali button battery zinc cream and a preparation method thereof. The invention solves the problem of pollution of lead and mercury in a battery in the prior art. The invention adopts the technical scheme that: the lead-free and mercury-free alkali button battery zinc cream is characterized in that the zinc cream is formed by mixing and stirring zinc powder mixture and electrolyte; and the zinc powder mixture is formed by mixing lead-free and mercury-free zinc powder, polyacrylic acid, polyacrylic ester and indium hydroxide. Compared with the prior art, the invention has the following advantages that: the prepared zinc cream does not contain lead and mercury and has good explosion-proof function, and the preparation method is simple and has high production efficiency.
Owner:东莞市天球实业有限公司
Nickel-metal hydride battery
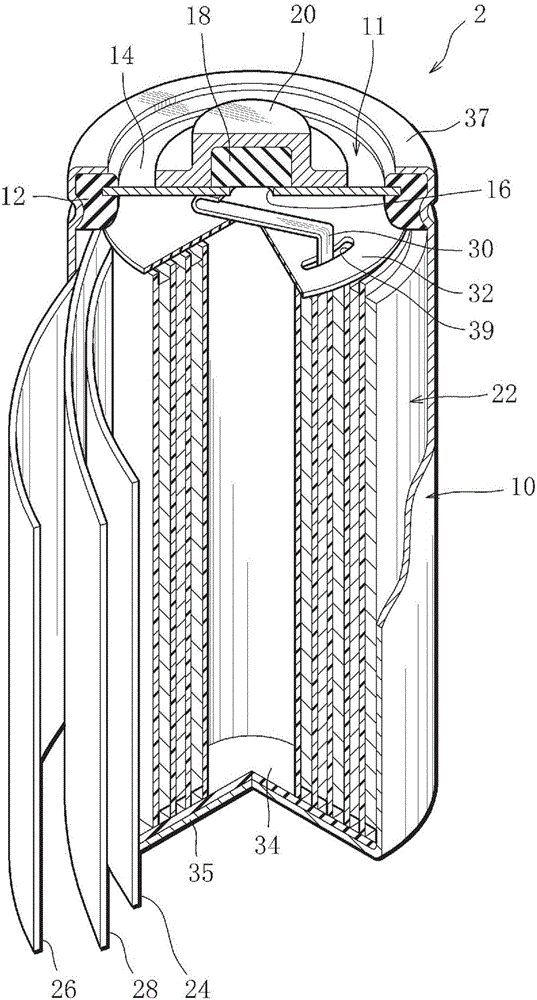
ActiveCN101292388ADoes not degrade cycle life characteristicsInhibition of self-dischargeNegative electrodesPositive electrodesFiberNickel oxide hydroxide
Owner:FDK CORP
Nickel hydrogen secondary battery
ActiveCN106133957AImprove simplicityFully utilized capacityCell electrodesNickel accumulatorsRare-earth elementEngineering
Owner:FDK CORP
Combined type lead-acid battery restorer
InactiveCN104779418ANo need to worry about damageLower internal resistanceLead-acid accumulatorsWaste accumulators reclaimingLead sulfateElectric power
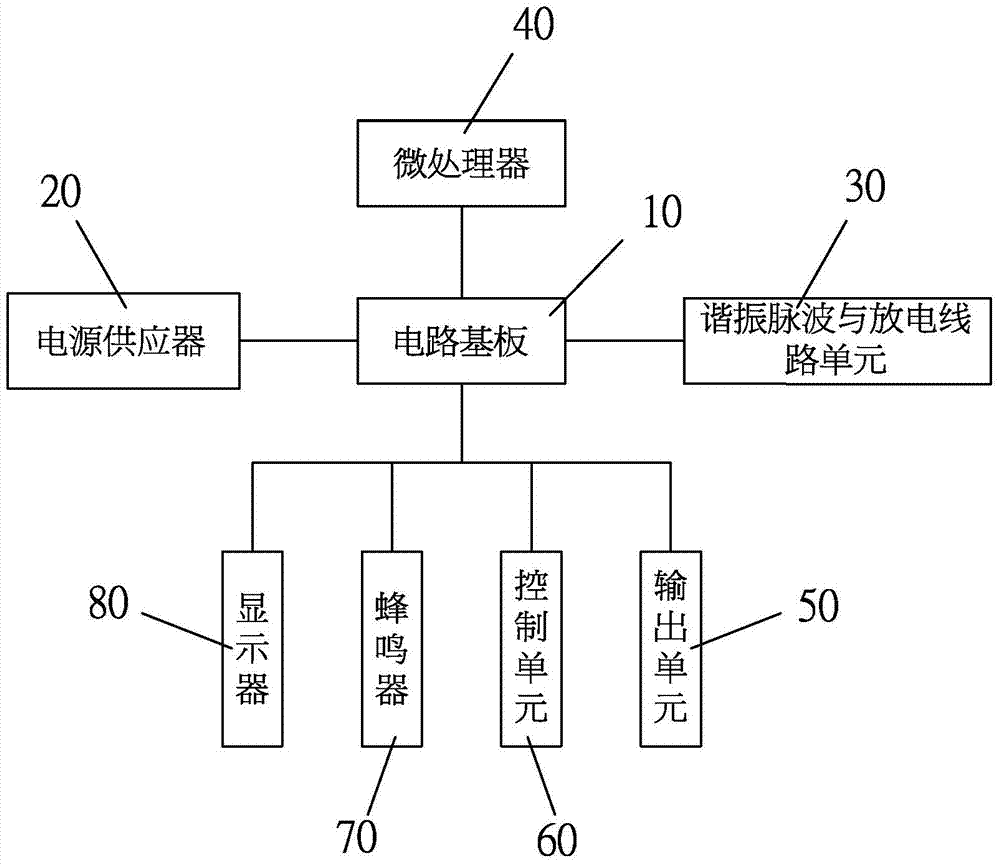
A combined type lead-acid battery restorer comprises a circuit board, a power supply, at least one resonant pulse unit, at least one discharge line unit, at least one microprocessor and an output unit. The power supply is arranged on the circuit board and is electrically connected to an external power supply, and supplies power to the circuit board. The at least one resonant pulse unit and the at least one discharge line unit are arranged on the circuit board. The at least one microprocessor is arranged on the circuit board, and the interior of the microprocessor is provided with a first procedure in advance, and the first procedure is a positive / negative frequency conversion pulse procedure. The output unit is provided with a positive electrode electric clamp and a negative electrode electric clamp, the positive electrode electric clamp is clamped on the positive electrode of a lead-acid battery with lead sulfate crystals, the negative electrode electric clamp is clamped on the negative electrode of the lead-acid battery with lead sulfate crystals, the lead-acid battery is detected by means of the microprocessor, and the resonant pulse unit and the discharge line unit output at least one positive / negative frequency conversion pulse through the output unit, the positive / negative frequency conversion pulse and the lead sulfate crystals in the lead-acid battery resonate, and therefore the lead sulfate crystals are crushed.
Owner:杨铭程
Alkaline storage battery
InactiveCN101179137AInhibition of self-dischargeSuppression of self-discharge characteristicsCell electrodesNickel accumulatorsSelf-dischargeMaterials science
The present invention relates to an alkaline accumulator; wherein, at least one of the surface of the membrane, the positive plate and the negative plate contains metallic compound; at least one of the positive plate and the negative plate contains the dissolvable metal; and the metallic compound makes the dissolvable metal dissolved from the alkaline electrolyte be separated out at the surface of the membrane, the surface or the inside of the positive plate, or the surface of the inside of the negative plate. Consequently, an alkaline accumulator which promises seldom self discharge and has long service life can be available.
Owner:PANASONIC CORP
Battery
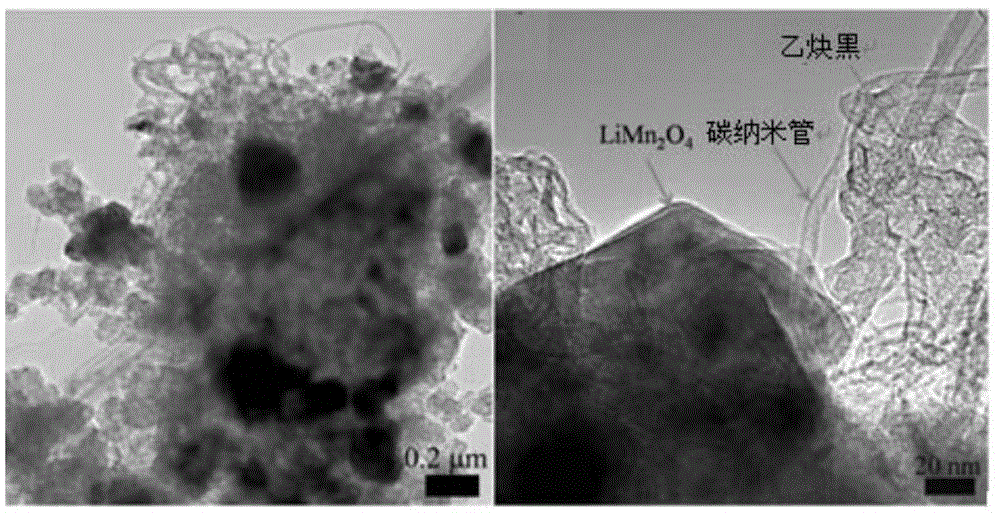
InactiveCN105449294AImprove stabilityImprove antioxidant capacityCell electrodesSecondary cellsMass ratioCarbon nanotube
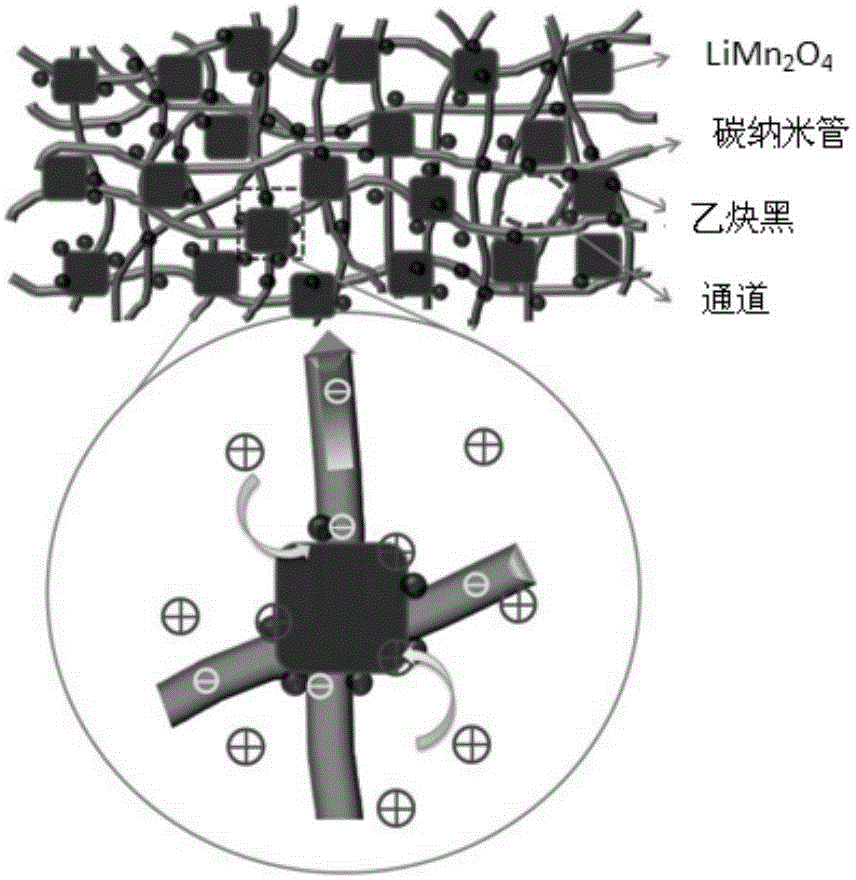
The invention provides a battery, which includes an anode, a cathode, and an electrolyte solution. The anode includes a positive active material able to realize reversible separation-embedding of first metal ions. The electrolyte solution consists of first metal ions and second metal ions, in the process of charging and discharging, the first metal ions can achieve reversible separation-embedding at the anode, the second metal ions can be reduced and deposited into second metal and the second metal can be reversibly oxidized and dissolved into second metal ions. Specifically, the anode also includes a composite conductive agent containing carbon nanotubes and acetylene black, the composite conductive agent and the positive active material are in a mass ratio of 0.005-0.3, and the carbon nanotubes and acetylene black are in a mass ratio of 0.25-1.25. The invention can improve the self-discharge problem of batteries, and the provided battery has long cycle life, excellent rate capability and high specific capacity.
Owner:POSITEC POWER TOOLS (SUZHOU) CO LTD +1
Non-aqueous electrolyte and non-aqoue electrolyte secondary cell
InactiveCN1373529AInhibit swellingInhibition of self-dischargeOrganic chemistryCell electrodesHigh temperature storageSolvent
The object of the present invention is to provide a non-aqueous electrolyte, which can suppress gas generation during high-temperature storage, prevent expansion of casing packaging materials, and can also suppress self-discharge under high-temperature environments, and has improved charge-discharge cycle characteristics. The non-aqueous solvent in the non-aqueous electrolyte includes ethylene carbonate, propylene carbonate, γ-butyrolactone, and the aforementioned ethylene carbonate, the aforementioned propylene carbonate, and the aforementioned γ-butyrolactone as the fourth component. Solvents other than lactones, corresponding to the aforementioned non-aqueous solvents as a whole, when the proportions of ethylene carbonate, propylene carbonate, γ-butyrolactone and the aforementioned fourth component are respectively recorded as x volume %, y volume %, When z volume % and p volume %, the aforementioned x, the aforementioned y, the aforementioned z and the aforementioned p respectively satisfy 15≤x≤50, 2≤y≤35, 30≤z≤85, 0
Owner:KK TOSHIBA
Binder composition for electrode
ActiveCN103140970AReduce adverse effectsImprove securityHybrid capacitor electrodesSecondary cellsMolecular orbitalPolymer chemistry
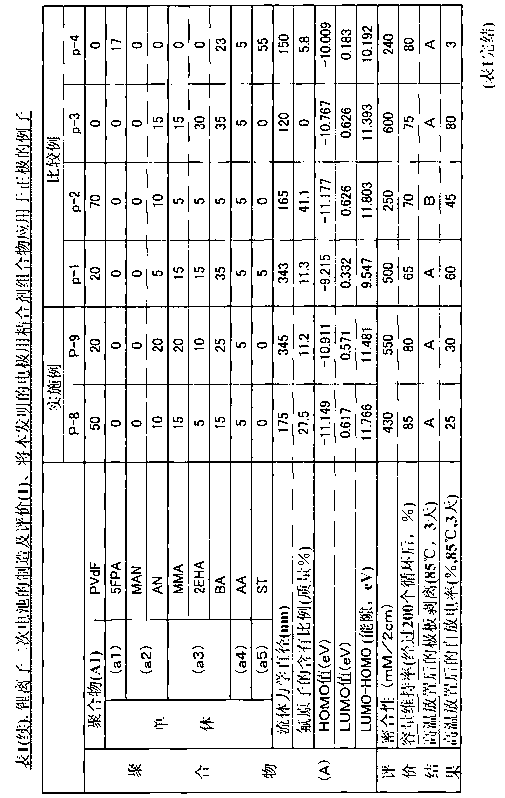
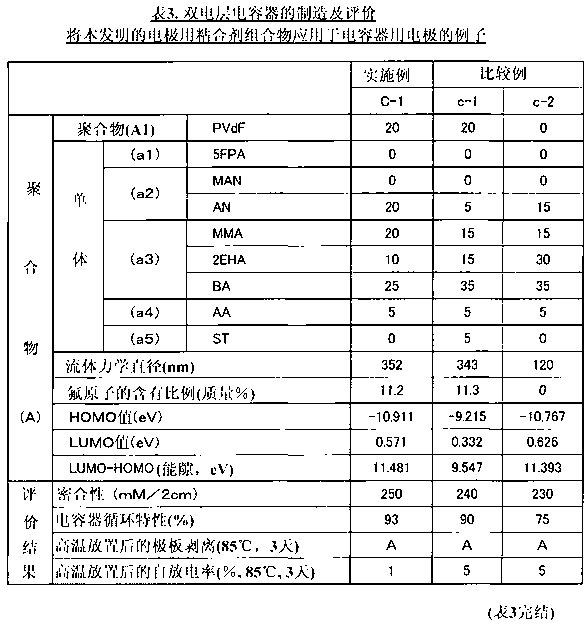
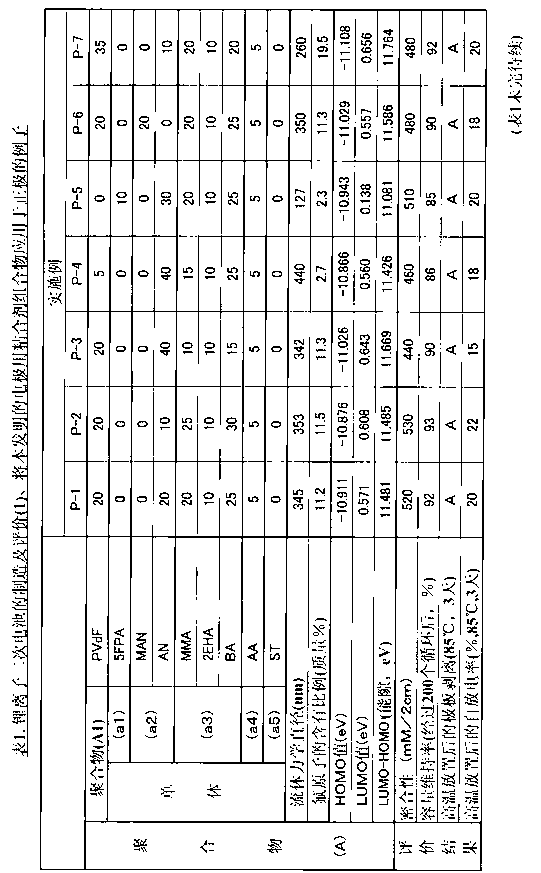
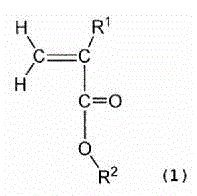
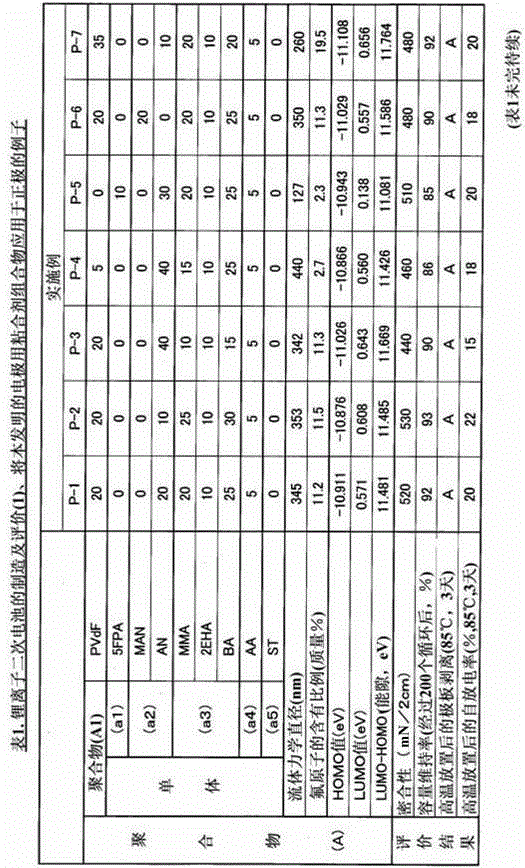
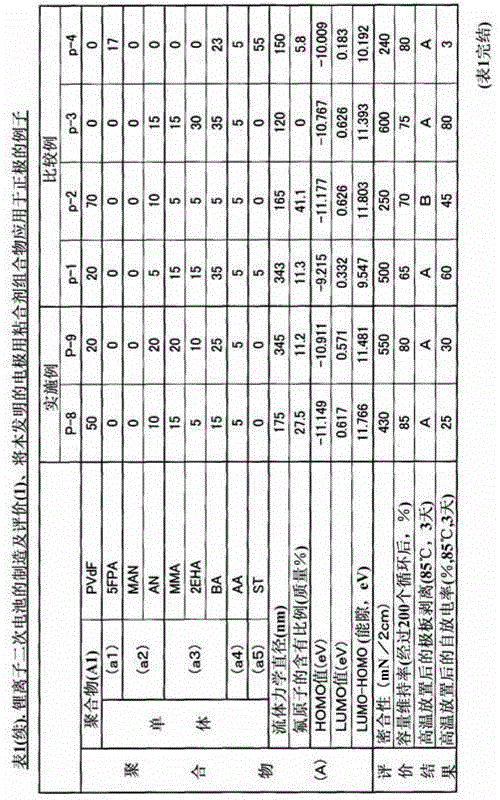
The present invention relates to a binder composition for an electrode containing a polymer and an aqueous vehicle. The binder composition is characterized in that the polymer is a polymer (A) with the HOMO level calculated according to the semiempirical molecular orbital method being -10 eV or lower, the difference between the LUMO level calculated according to the semiempirical molecular orbital method and the HOMO level being 10.5 eV or larger, and the content of fluorine atoms measured by combustion ion chromatography being 2 to 30 mass%. An electrochemical device obtained by using this binder composition for an electrode can maintain adhesion even under a high-temperature environment and has highly suppressed self discharge under a high-temperature environment.
Owner:株式会社引能仕材料
Nickel-metal hydride battery
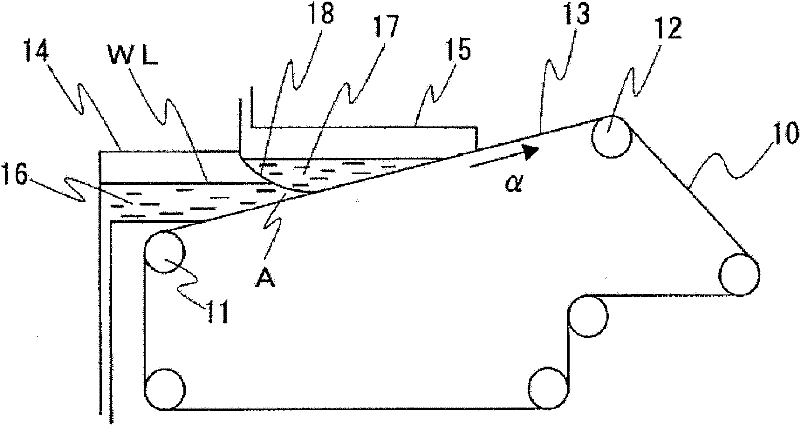
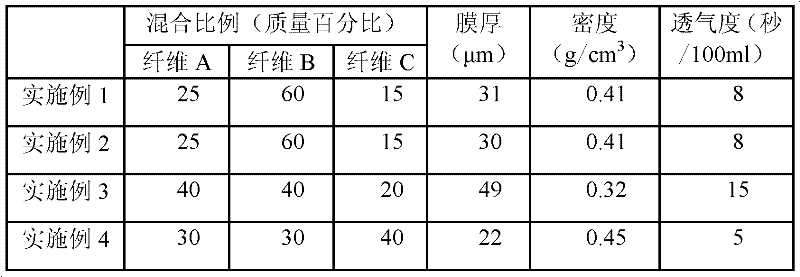
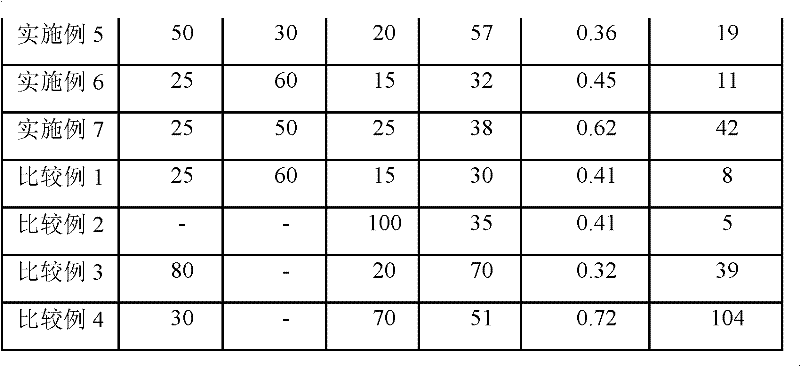
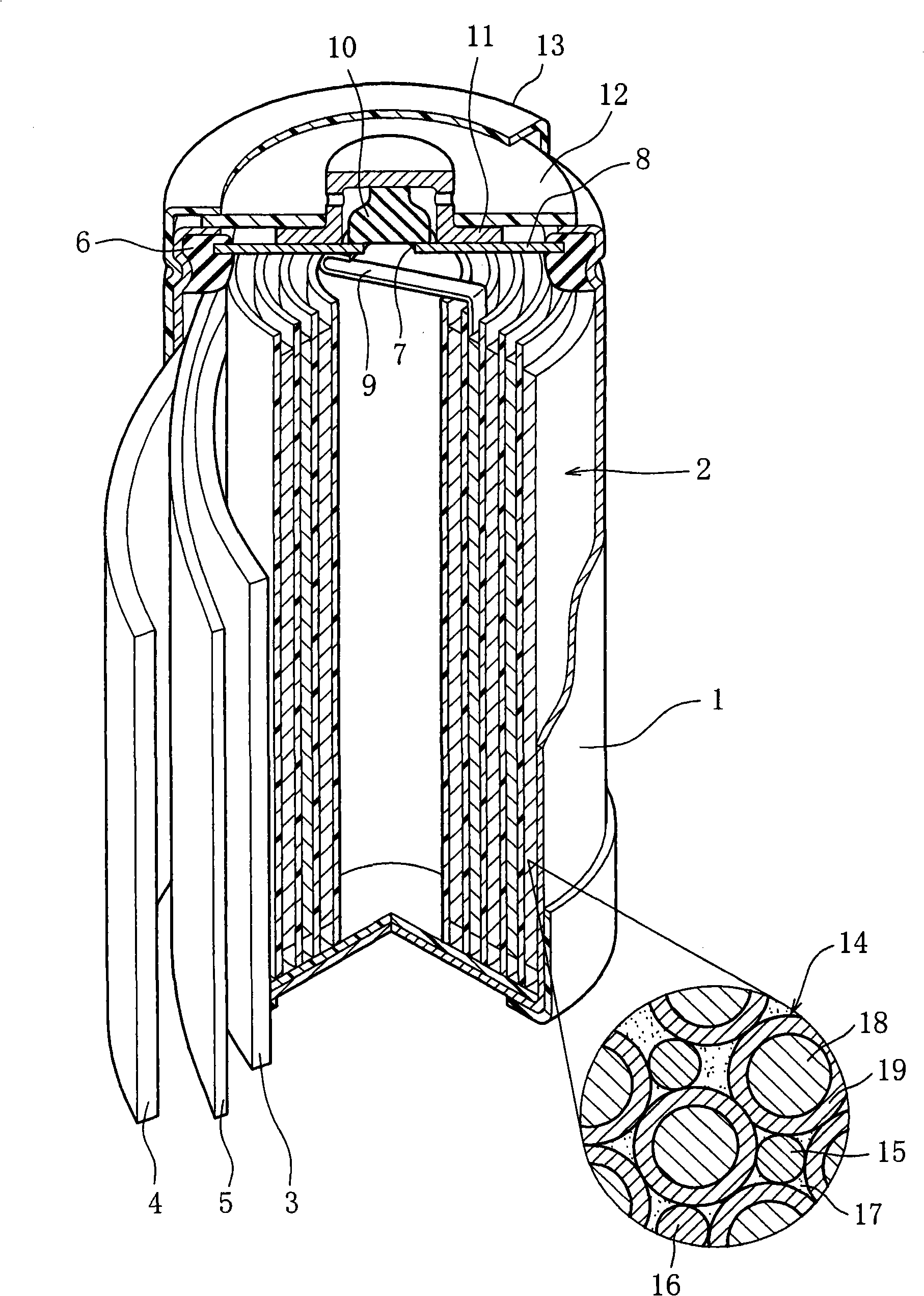
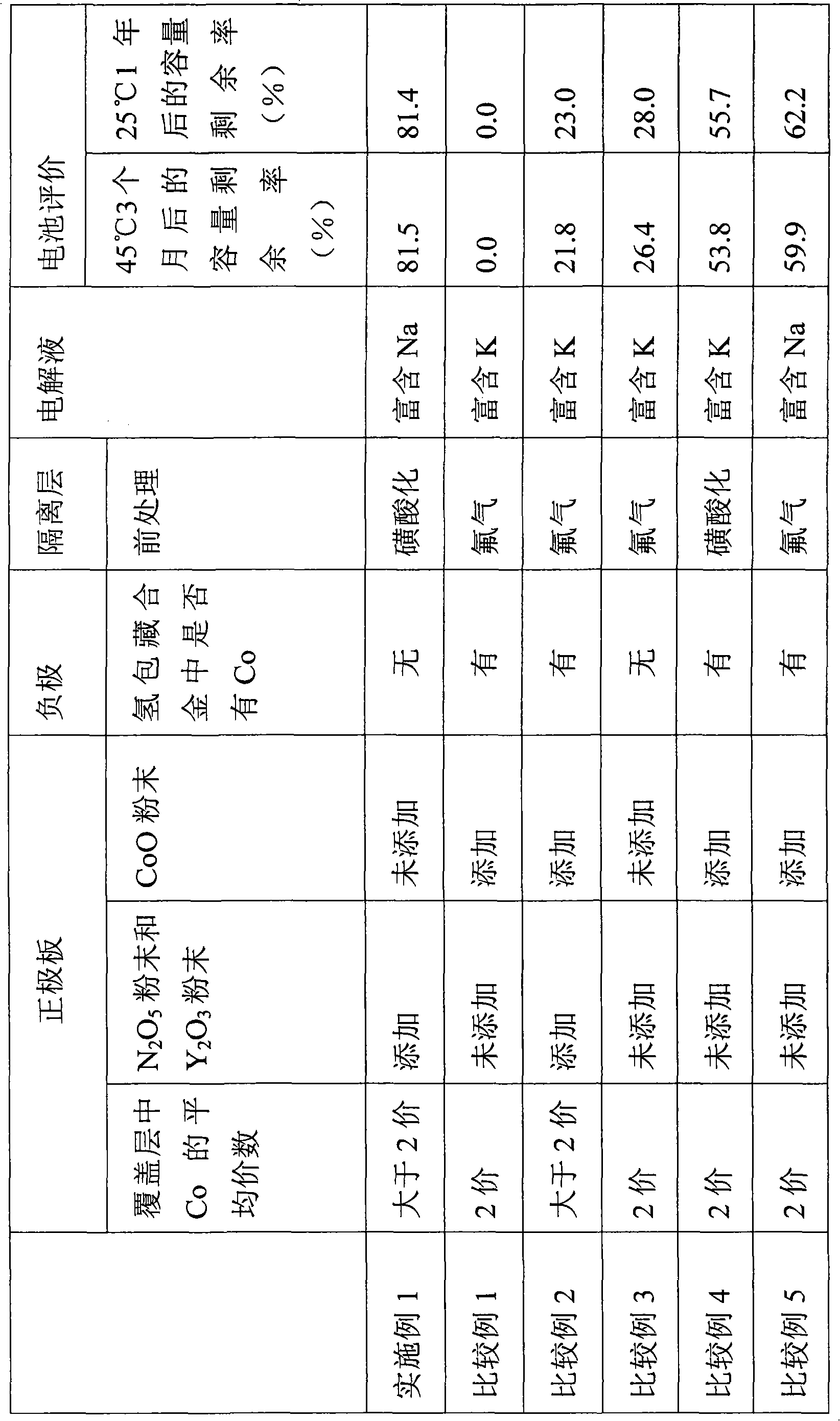
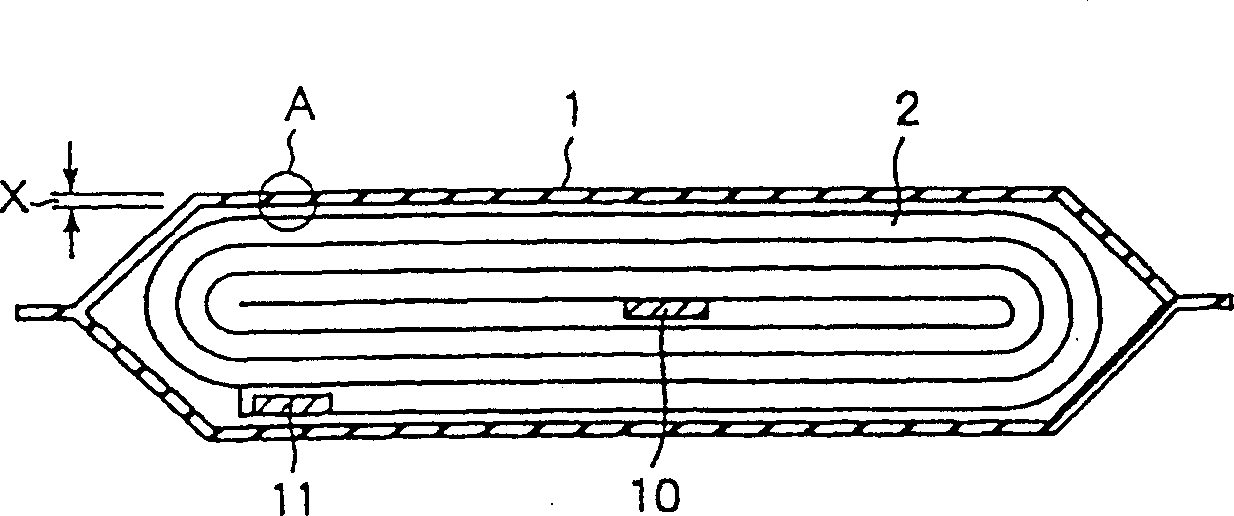
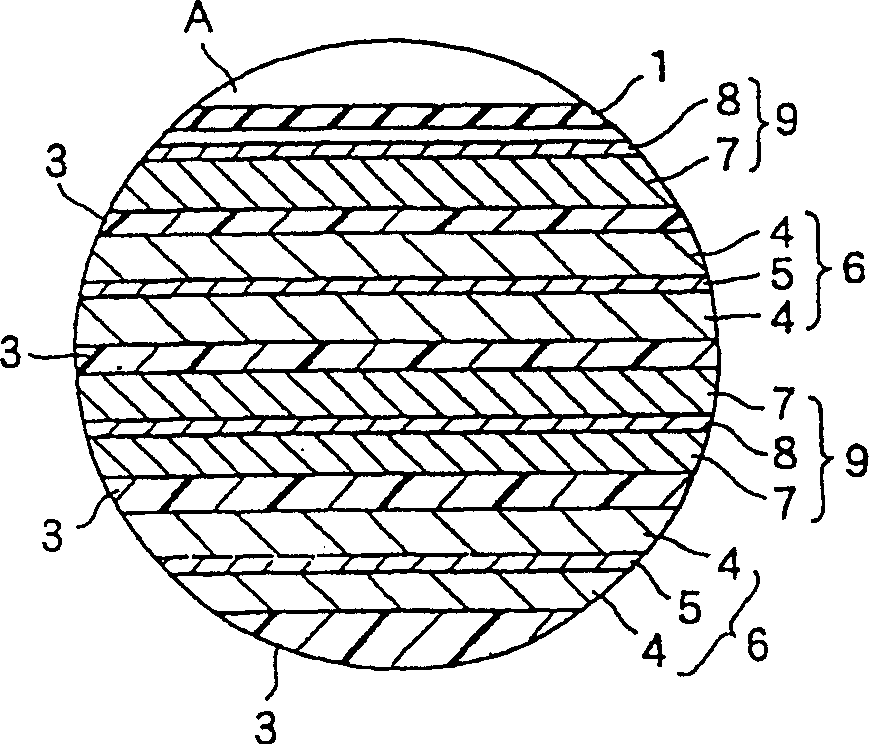
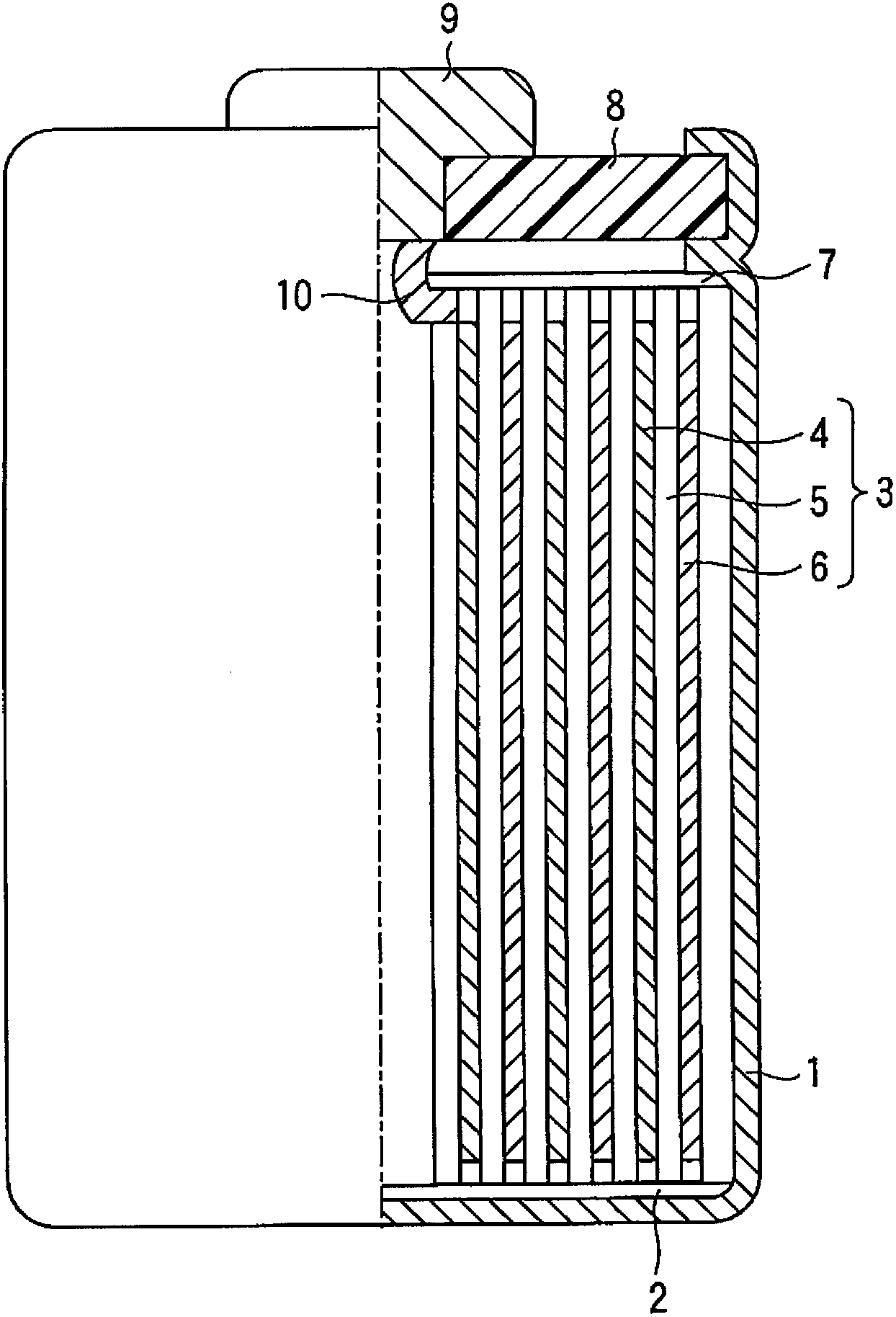
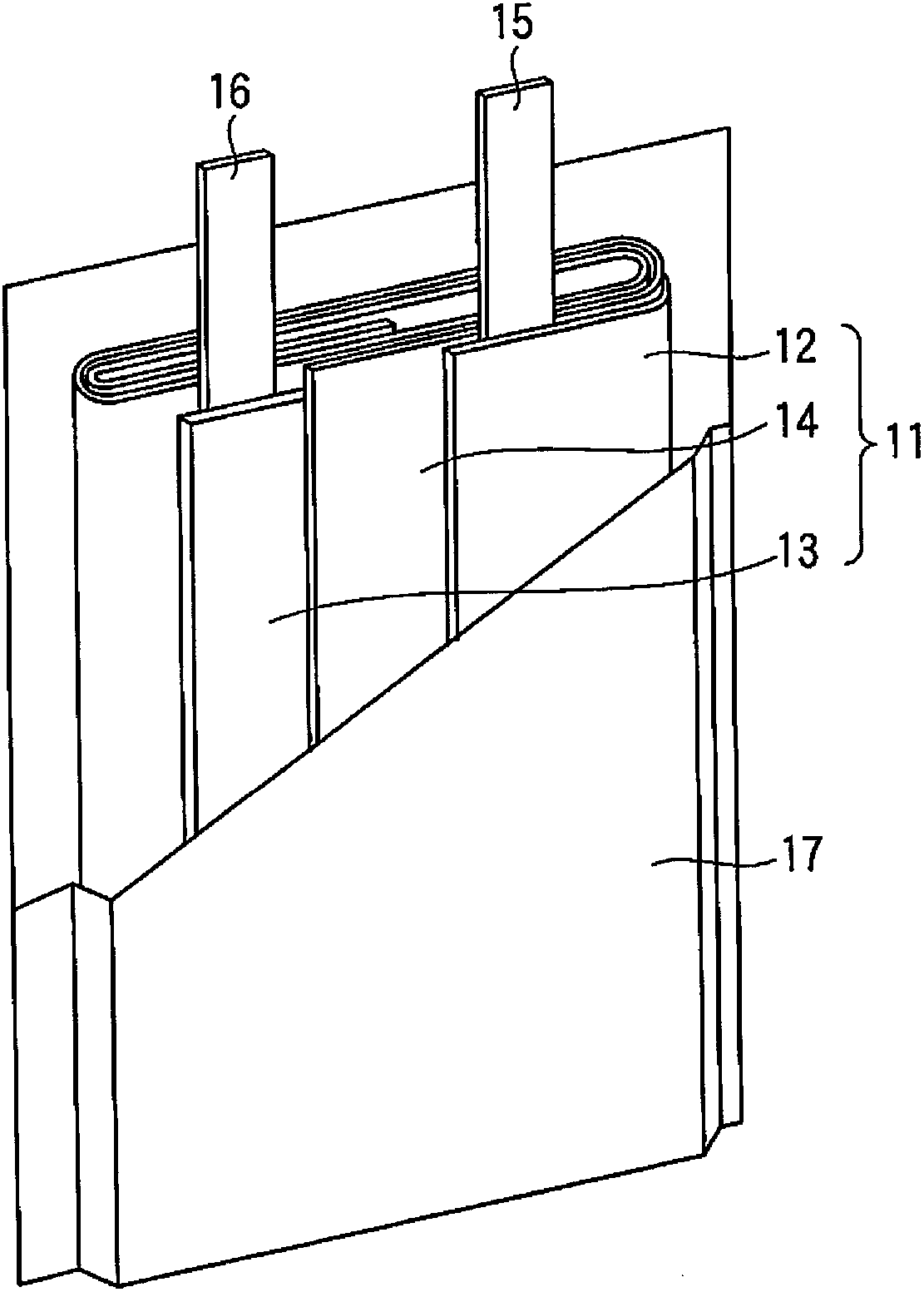
InactiveCN101297432AInhibition of self-dischargeSuppression of operating voltage dropFinal product manufactureElectrode carriers/collectorsElectrolytic agentSulfone
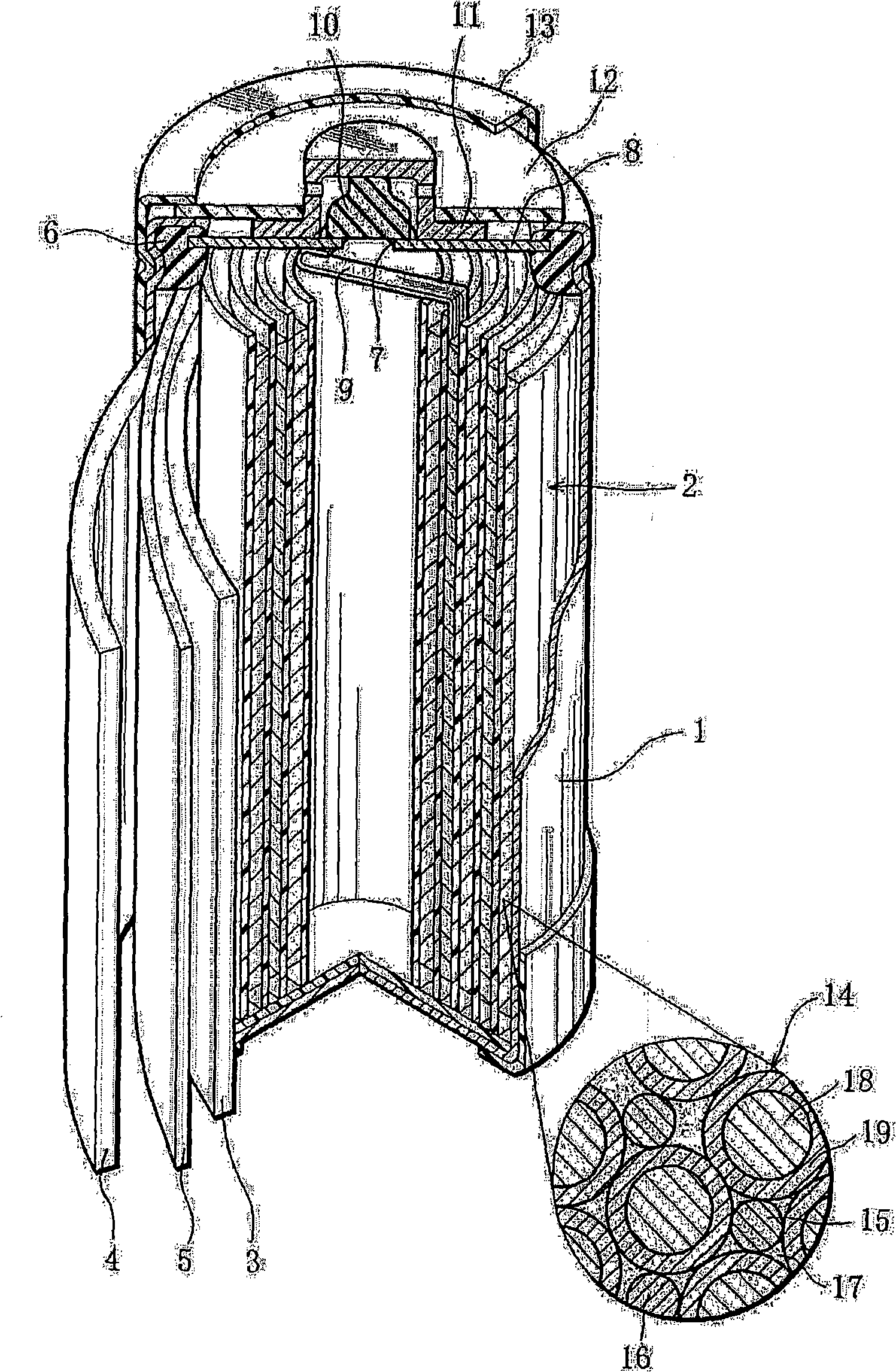
A positive electrode plate (3) of a nickel-metal hydride battery contains nickel hydroxide particles (18), a coating layer (19) covering at least a part of the surface of each nickel hydroxide particle (18) and mainly composed of a cobalt compound having an average valence of cobalt more than 2, and Nb-based particles (15) and Y-based particles (16) distributed among the nickel hydroxide particles (18). A negative electrode plate (4) contains a hydrogen storage alloy having a Co content of not more than 2.0% by mass, a separator (5) contains a fiber having a sulfone group, and an alkaline electrolyte solution contains sodium hydroxide as the main solute.
Owner:FDK CORP
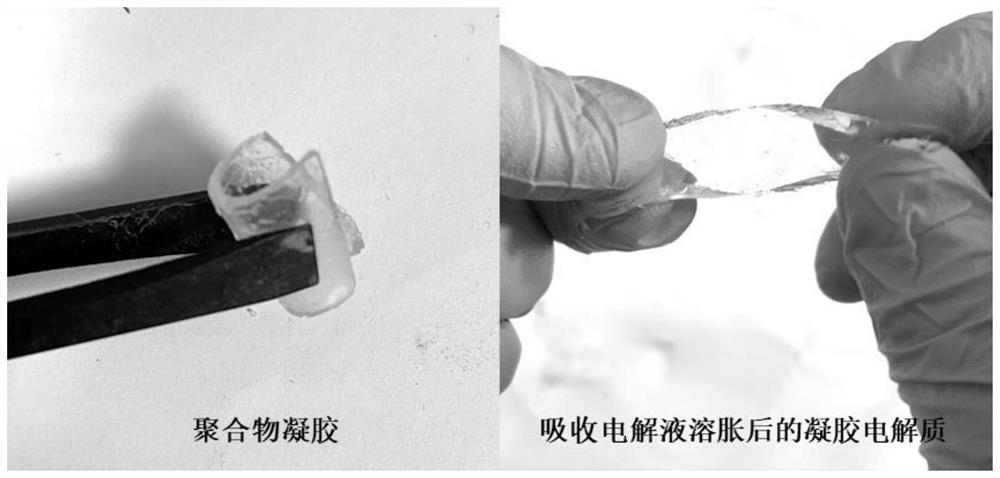
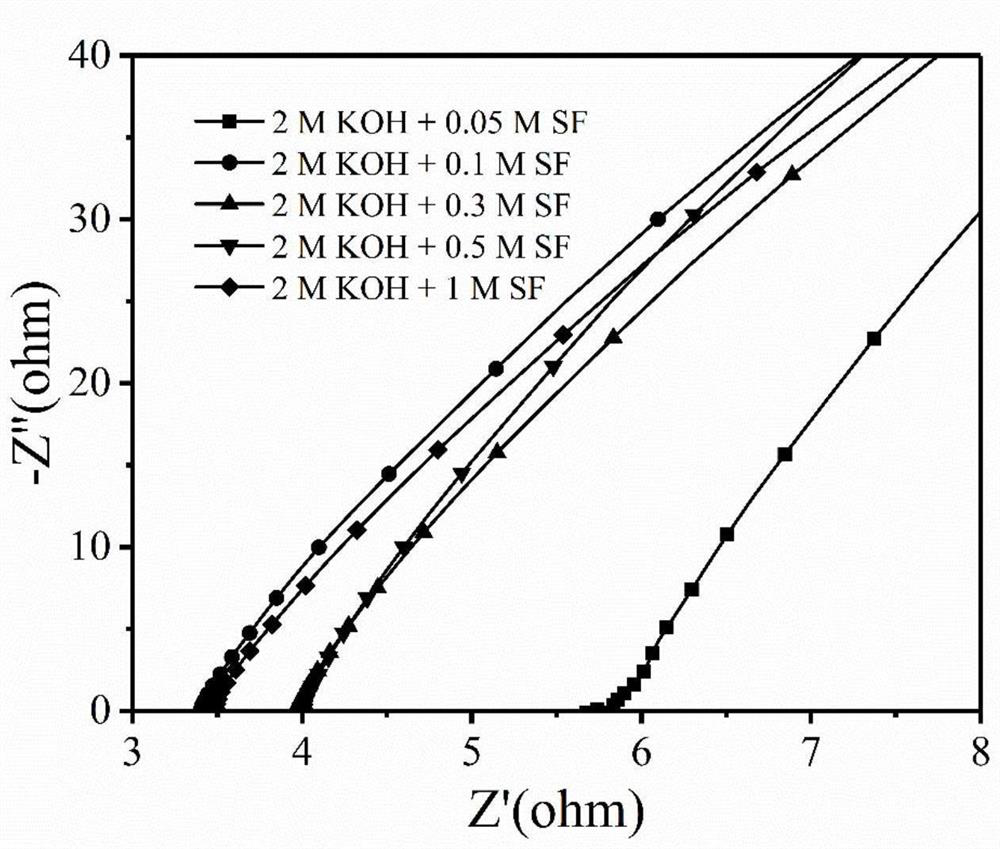
High-stability gel electrolyte for zinc-air battery, and preparation method of high-stability gel electrolyte
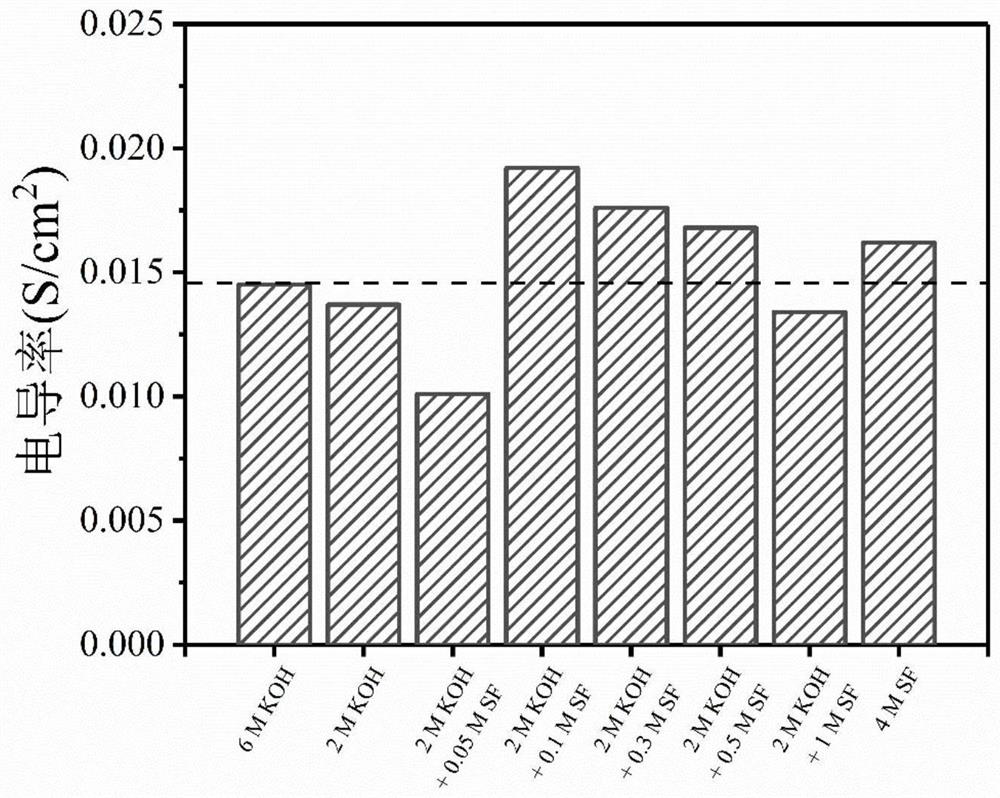
PendingCN112599892AImprove hydroxide ion conductivityReduce the concentration of alkaliFuel and secondary cellsElectrolyte immobilisation/gelificationAlkali saltPolymer gel
The invention discloses a high-stability gel electrolyte for a zinc-air battery and a preparation method of the high-stability gel electrolyte, and belongs to the field of new energy materials, wherein a polymer gel adsorbs a low-concentration alkaline electrolyte aqueous solution containing an organic weak-acid strong-alkali salt additive and then is swollen to prepare the gel electrolyte. According to the invention, the solutes of the electrolyte are potassium hydroxide, sodium hydroxide and other strong alkalis, organic weak acid and strong alkali salt additives (formate, acetate, benzoateand the like) and organic acid radicals, so that the electrolyte can still provide high-concentration hydroxyl ions required by electrode reaction under the condition of lower alkali concentration; the alkali concentration of the gel electrolyte prepared by the method is greatly reduced, so that the corrosion of the high-concentration alkali electrolyte to a zinc negative electrode is remarkably reduced; and the quasi-solid-state zinc-air battery using the gel electrolyte inhibits the self-discharge phenomenon of the battery while ensuring the stable operation of the battery.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS +1
Electrode, secondary battery, battery pack, and vehicle
PendingCN112531143ALower resistanceInhibition of self-dischargeNegative electrodesCells structural combinationComposite oxideBattery cell
The present invention provides an electrode of a secondary battery capable of achieving low resistance and suppressing self-discharge, a secondary battery provided with the electrode, a battery pack provided with the secondary battery, and a vehicle provided with the battery pack. According to one embodiment, an electrode is provided. The electrode includes a current collector, a first layer formed on the current collector, and a second layer formed on at least part of the first layer. The first layer contains a monoclinic niobium titanium composite oxide. The second layer contains lithium titanate having a spinel structure. A porosity P2 of the second layer is within a range from 30% to 80%.
Owner:KK TOSHIBA
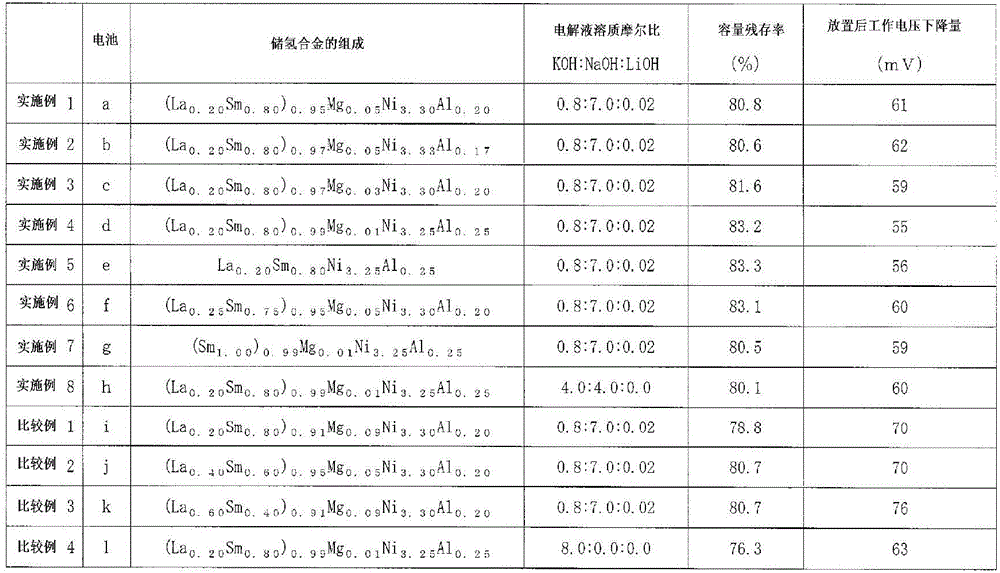
Hydrogen storage alloy powder and nickel hydrogen secondary battery using this hydrogen storage alloy powder
ActiveCN107408683AEasy to useGuaranteed voltageTransportation and packagingMetal-working apparatusElectrolytic agentElectrical battery
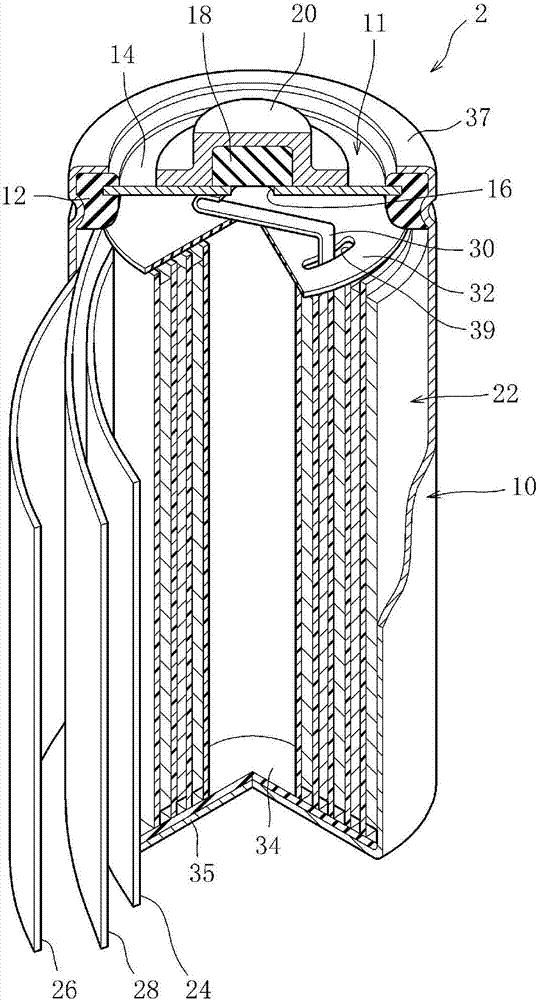
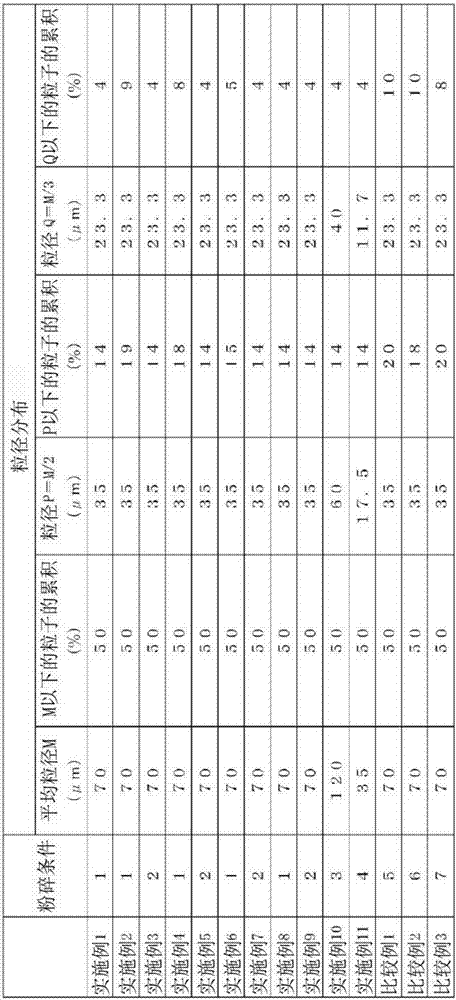
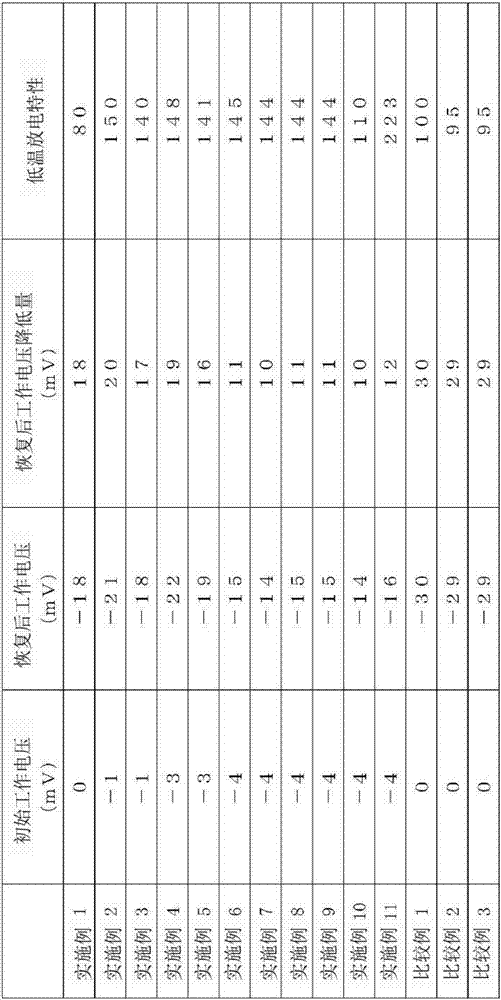
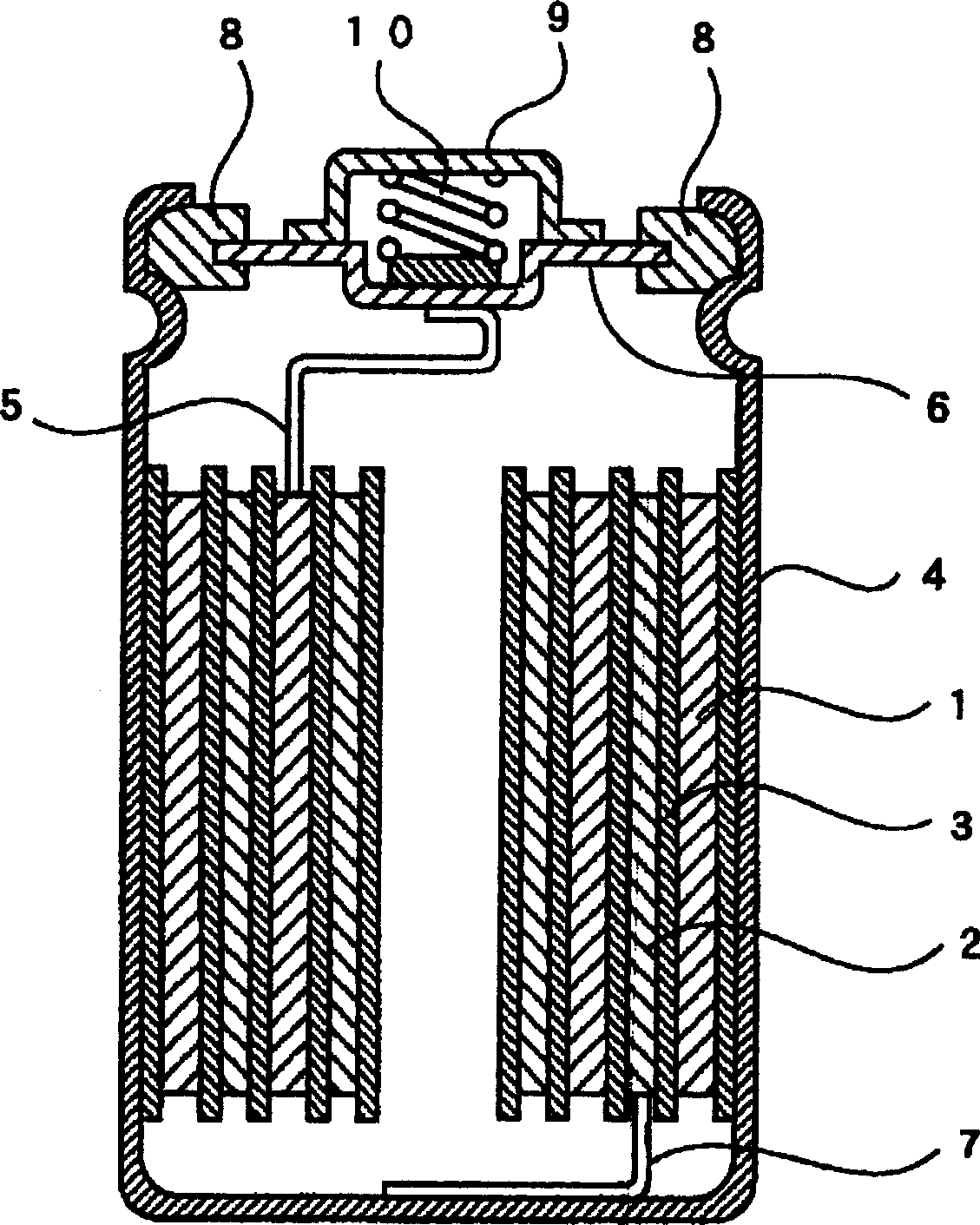
This nickel hydrogen secondary battery 2 is provided with: an outer casing can 10; and an electrode group 22 that is contained within the outer casing can 10 together with an alkaline electrolyte solution in a hermetically sealed state. The electrode group 22 is composed of a positive electrode 24 and a negative electrode 26, which are superposed on each other with a separator 28 being interposed therebetween. The negative electrode 26 contains a hydrogen storage alloy powder that is an aggregate of hydrogen storage alloy particles. If M is the average particle diameter of the hydrogen storage alloy particles, P is the particle diameter equal to 1 / 2 of M, and Q is the particle diameter equal to 1 / 3 of M, the content of the particles having particle diameters of P or less is less than 20% by mass of the whole hydrogen storage alloy powder, and the content of the particles having particle diameters of Q or less is less than 10% by mass of the whole hydrogen storage alloy powder.
Owner:FDK CORP
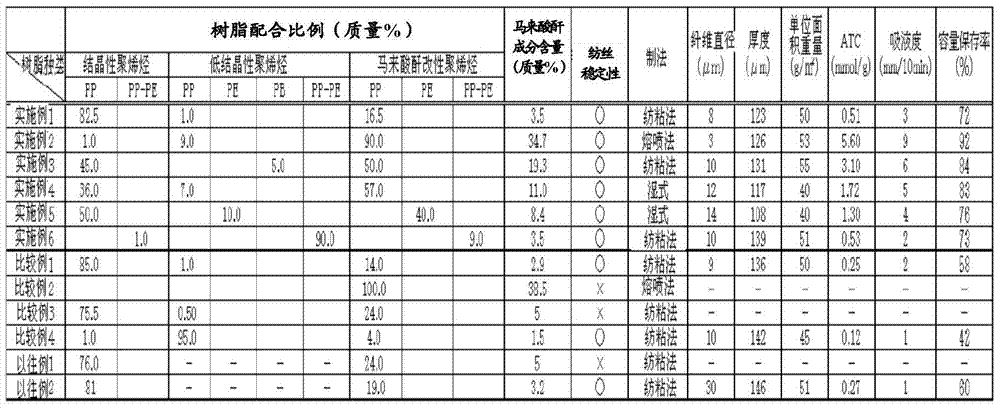
Fiber using olefin resin, nonwoven fabric using same, and separator for alkali storage battery
InactiveCN104520478AInhibition of self-dischargeMonocomponent polypropylene artificial filamentCell component detailsPolymer sciencePolyolefin
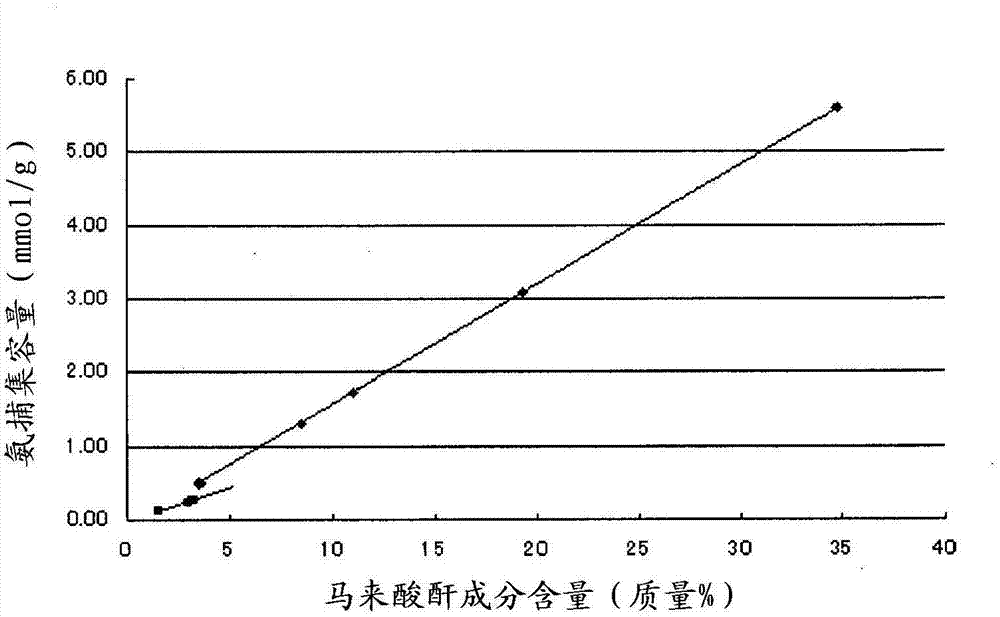
There is provided a low-cost alkaline storage battery separator capable of implementing stable fiber spinning and excellent ammonia trapping function so as to control self-discharge. Spinning fiber using resin including 1.0 mass % or greater of low crystalline polyolefin, crystalline polyolefin and maleic anhydride-modified polyolefin corresponding to a maleic anhydride component content of 3.5 to 35 mass % in the fiber assures stability during fiber spinning. Moreover, making a nonwoven fabric using the fiber act as a separator allows a high level of ammonia trapping function and controls self-discharge of the alkaline storage battery.
Owner:NIPPON KODOSHI
Non equeous electrolyte battery
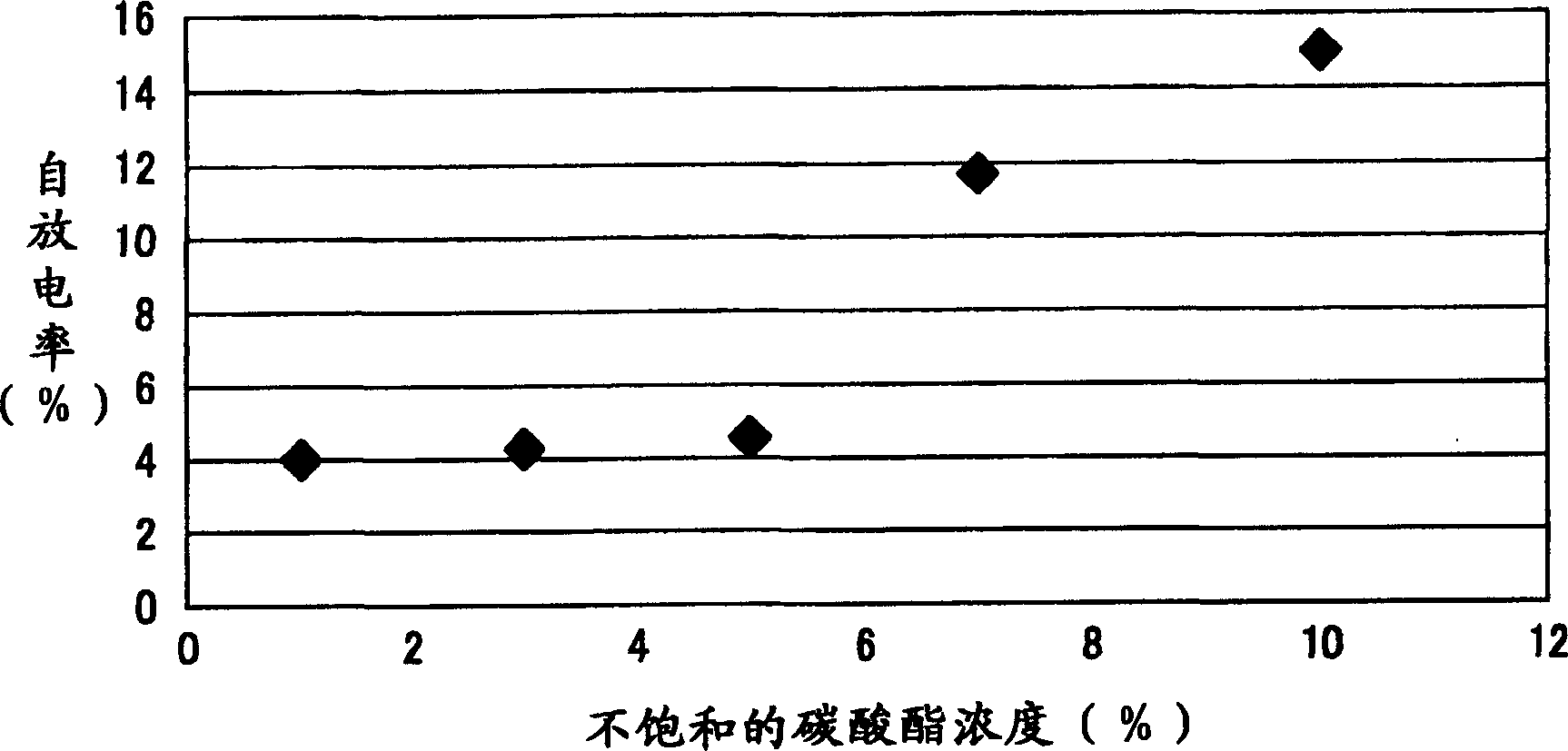
InactiveCN1442926AAvoid dissociationInhibition of self-dischargeFinal product manufactureOrganic electrolyte cellsAqueous solutionCarbonate
A nonaqueous electrolyte battery includes: a cathode using a composite compound of lithium and a transition metal as a positive electrode active material; an anode using a negative electrode active material capable of doping or extracting lithium; and a nonaqueous electrolyte sandwiched between the anode and the cathode. By dissolving LiMFm (where M is an element selected from As, B, P and Sb, and m is an integer ranging from 4 to 6) and LiCnF2n+1SO3 or LiN(CnF2n+1SO2)2 in the In the non-aqueous solvent of cyclic carbonate or non-cyclic carbonate, add more than or equal to 0.1 volume % and less than or equal to 5 volume % of unsaturated carbonate to obtain non-aqueous electrolyte solution, LiCnF2n+1SO3 or LiN (CnF2n+ The concentration of 1SO2)2 is greater than or equal to 1 wt% and less than 10 wt%. Therefore, self-discharge is suppressed, and storage resistance is improved.
Owner:SONY CORP
Nonaqueous electrolyte solution and nonaqueous secondary battery
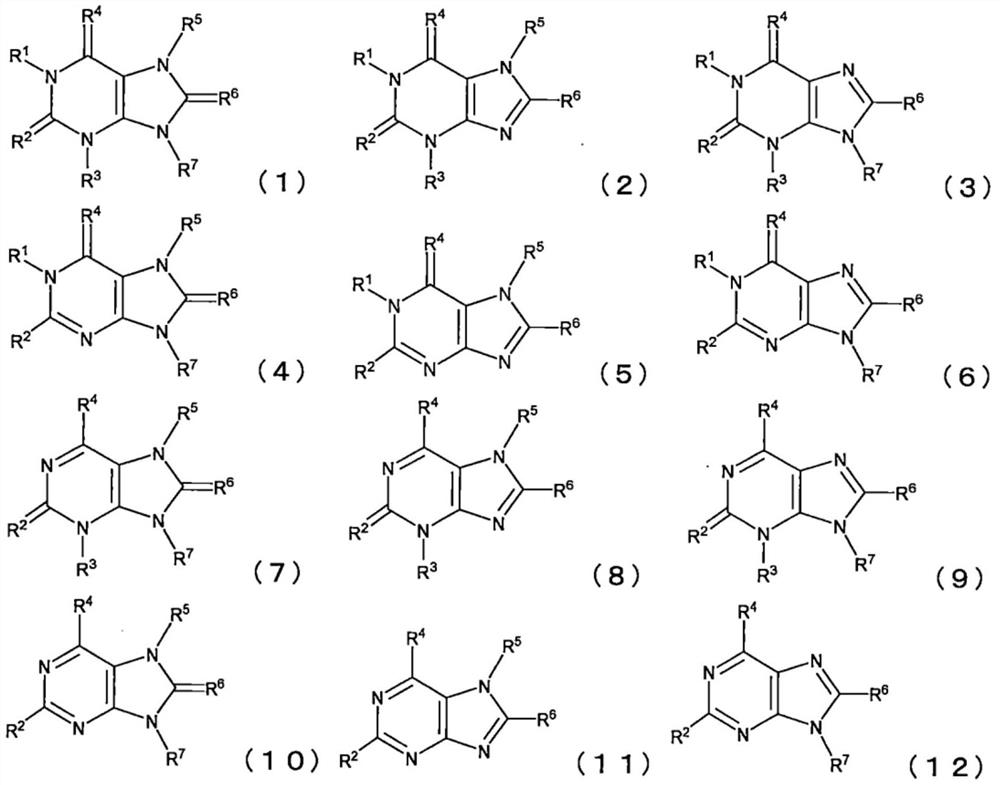
PendingCN112640180AInhibition of deterioration reactionInhibition of self-dischargeOrganic chemistryNegative electrodesPyrimidineElectrolyte
Provided is a nonaqueous electrolyte solution comprising a nonaqueous solvent containing acetonitrile in an amount of 5 to 95% by volume, a lithium salt, and at least one compound having a structure that satisfies the following requirements 1 to 5: 1. the compound is a condensed polycyclic heterocyclic compound; 2. a pyrimidine skeleton is contained in the condensed polycyclic heterocyclic ring; 3. at least three nitrogen atoms are contained in the condensed polycyclic heterocyclic ring; 4. at least five sp2 carbon atoms are contained in the condensed polycyclic heterocyclic ring; and 5. no hydrogen atom is bonded to the nitrogen atoms in the condensed polycyclic heterocyclic ring.
Owner:ASAHI KASEI KK
Manufacturing method of zinc nickel primary battery capable of high temperature storage
InactiveCN1753215AInhibition of self-dischargeGood heavy load discharge performanceCell electrodesPrimary cellsHigh temperature storageOxygen
The invention provides a Zn-Ni primary battery having excellent heavy-load discharging property even if stored for 3 weeks on the conditions of 60 deg. c and humidity of 90% RH. The invention contains anode, cathode, diaphragm and basic electrolyte, where the anode comprises MnO2 and NiOOH as active substances and at least one of the oxygen-containing compounds of Mg, Zn, Ti, Sc, Ce and Y and the cathode comprises Zn as active substance.
Owner:江苏省新动力电池及其材料工程技术研究中心有限公司
Non-aqueous electrolyte and non-aqueous electrolyte secondary cell
InactiveCN1204648CInhibit swellingInhibition of self-dischargeCell electrodesFinal product manufactureHigh temperature storageSolvent
Owner:KK TOSHIBA
Biotissue transdermal patch
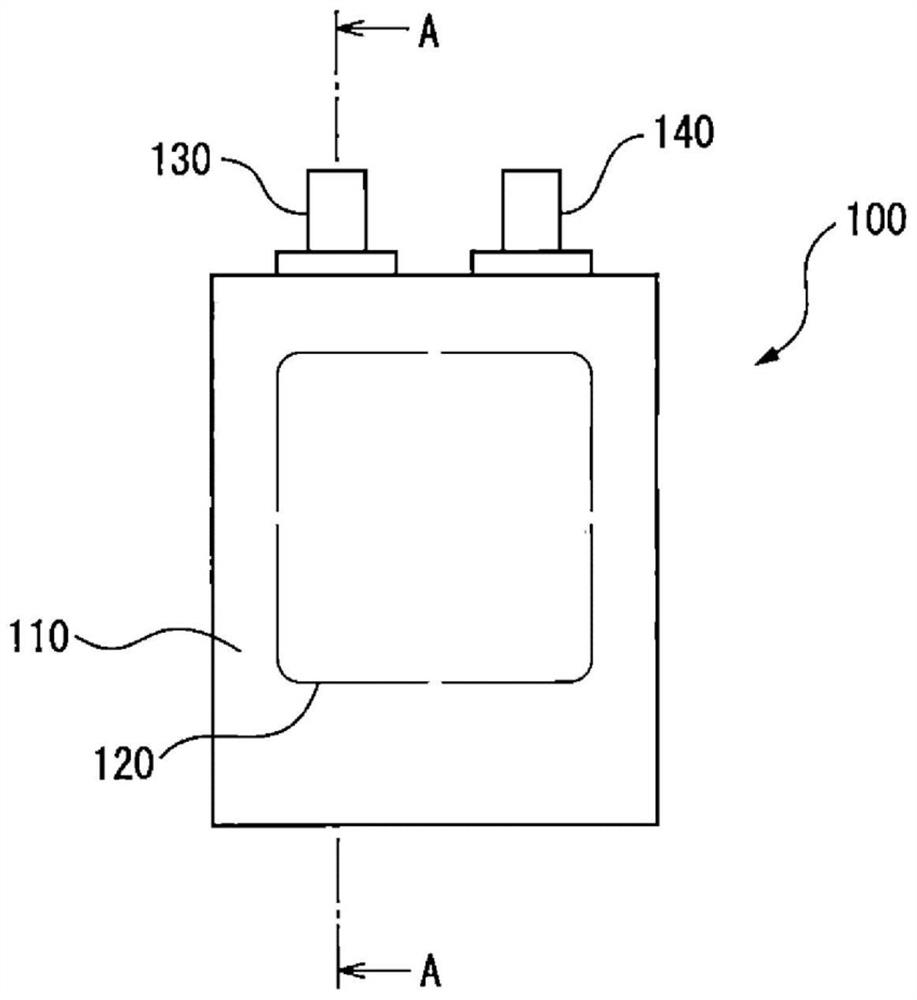
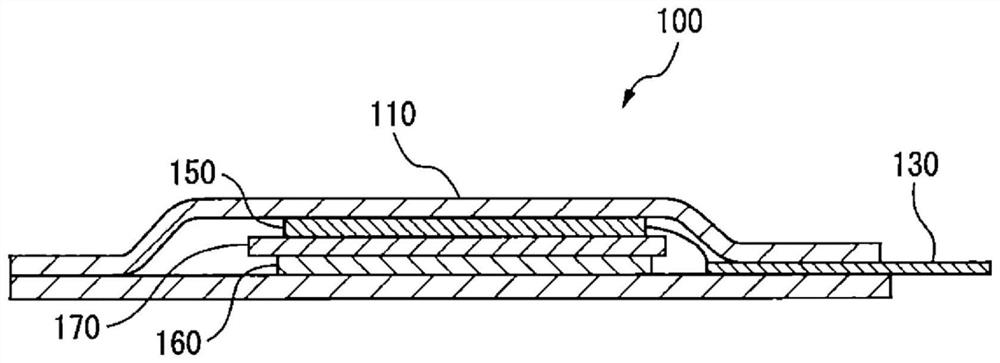
PendingCN110418662AInhibition of self-dischargeElectrotherapySheet deliveryCarbon nanofiberBiomedical engineering
A biotissue transdermal patch 1 houses a battery part 2 and an active ingredient 3 so as to prevent contact therebetween, causes the battery reaction to begin in the battery part 2 when the biotissuetransdermal patch 1 is in use by effecting contact between the battery part 2 and active ingredient 3, and adheres the battery part 2 to biotissue. Either carbonized bacterial cellulose or cellulose-derived carbon nanofibers are used in a positive terminal 201 of the battery part 2.
Owner:NIPPON TELEGRAPH & TELEPHONE CORP
Binder composition for electrode
ActiveCN103140970BReduce adverse effectsImprove securityHybrid capacitor electrodesSecondary cellsIon chromatographyCombustion
A binder composition for electrodes, comprising a polymer and an aqueous medium, wherein the polymer consists of a polymer (A) which has a HOMO value calculated by a semiempirical molecular orbital method of -10 eV or less, a difference between its LUMO value calculated by the semiempirical molecular orbital method and its HOMO value of 10.5 eV or more and a fluorine atom content measured by combustion ion chromatography of 2 to 30 mass%. An electrochemical device obtained from the above binder composition for electrodes of the present invention can retain adhesion even in a high-temperature environment and its self-discharge in a high-temperature environment is highly suppressed.
Owner:株式会社引能仕材料
Negative electrode for zinc-based flow battery, and battery and application thereof
ActiveCN112928282AReduced Coulombic efficiencyAvoid direct contactCell electrodesRegenerative fuel cellsElectrical batteryConductive materials
The invention provides a long-life zinc-based flow battery. A negative electrode of the battery comprises a three-dimensional conductive carbon material and an inert conductive material; and the thickness of the inert conductive material is 20-50% of that of the three-dimensional conductive carbon material. According to the negative electrode and the battery, a self-discharge reaction caused by contact between the positive active material and the negative active material of the battery can be effectively inhibited, the coulombic efficiency of the battery is improved, a reaction interface for inducing zinc deposition occurs in the electrode, and the coulombic efficiency of the zinc-based flow battery is improved.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Nickel-hydrogen battery
InactiveCN1481043AEfficient captureInhibition of self-dischargeAlkaline accumulator electrodesNickel accumulatorsNickel oxide hydroxideImpurity
A nickel metal hydride storage battery which includes a positive electrode containing nickel hydroxide as a active material, a negative electrode containing a hydrogen absorbing alloy which contains aluminum, a separator and an alkaline electrolyte, wherein a complex-forming agent which forms a complex with aluminum is included in the negative electrode.
Owner:SANYO ELECTRIC CO LTD
Zinc electrode containing combined zinc corrosion inhibitor and zinc-nickel battery using zinc electrode
PendingCN114792780AHigh capacity retentionStrong charge retentionAlkaline accumulator electrodesNickel accumulatorsCharge retentionIndium
The invention provides a zinc electrode containing a combined zinc corrosion inhibitor. The material of the zinc electrode comprises the following components in percentage by mass: 0.001-1% of an organic corrosion inhibitor; 0.001 to 1% of indium oxide; 0-5% of bismuth oxide; 0.1 to 30% of conductive ceramic; 0.5%-5% of PTFE (polytetrafluoroethylene); 0.1%-3% of a thickening agent; the balance is zinc oxide; wherein an F-containing surfactant is selected as the organic corrosion inhibitor. The invention also provides a zinc-nickel battery of the zinc electrode and a zinc-nickel battery simultaneously applying the organic corrosion inhibitor and indium oxide. The zinc electrode containing the combined zinc corrosion inhibitor has the advantages of high capacity retention ratio, strong charge retention capability and capability of inhibiting self discharge.
Owner:山东合泰新能源有限公司
Novel boron-containing sulfonate non-aqueous electrolyte additive and lithium ion battery prepared from same
ActiveCN114188608AImprove thermal stabilityNot easy to decomposeSecondary cellsBatteriesElectrolytic agentOrganosolv
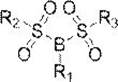
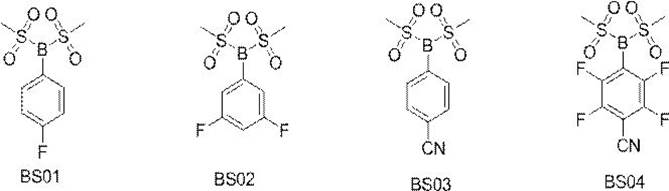
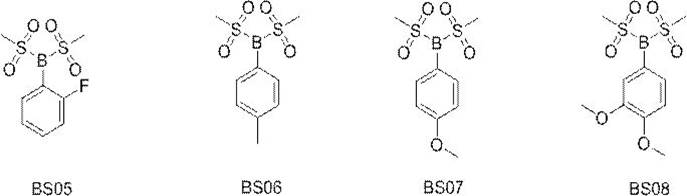
The invention relates to a novel boron-containing sulfonate non-aqueous electrolyte additive. The structural formula of the non-aqueous electrolyte additive is shown as a formula I, wherein the formula IR1 is selected from one of phenyl, biphenyl, fluorophenyl, cyano-containing phenyl and fluorobiphenyl; r2 and R3 are the same or different and are respectively and independently selected from one of methyl and trifluoromethyl. The boron-containing sulfonate non-aqueous electrolyte additive is applied to an electrolyte of a lithium ion battery, the electrolyte comprises a non-aqueous organic solvent, an electrolyte lithium salt and an additive, and the additive comprises the boron-containing sulfonate non-aqueous electrolyte additive. When the non-aqueous electrolyte additive is applied to the lithium ion battery, the internal resistance of the battery can be obviously reduced, and the low-temperature circulation, high-rate normal-temperature circulation, high-temperature circulation and expansion after high-temperature storage of the battery are obviously improved.
Owner:VALIANT CO LTD
An oxygen ion conductive metal-metal oxide molten salt secondary battery and its preparation method
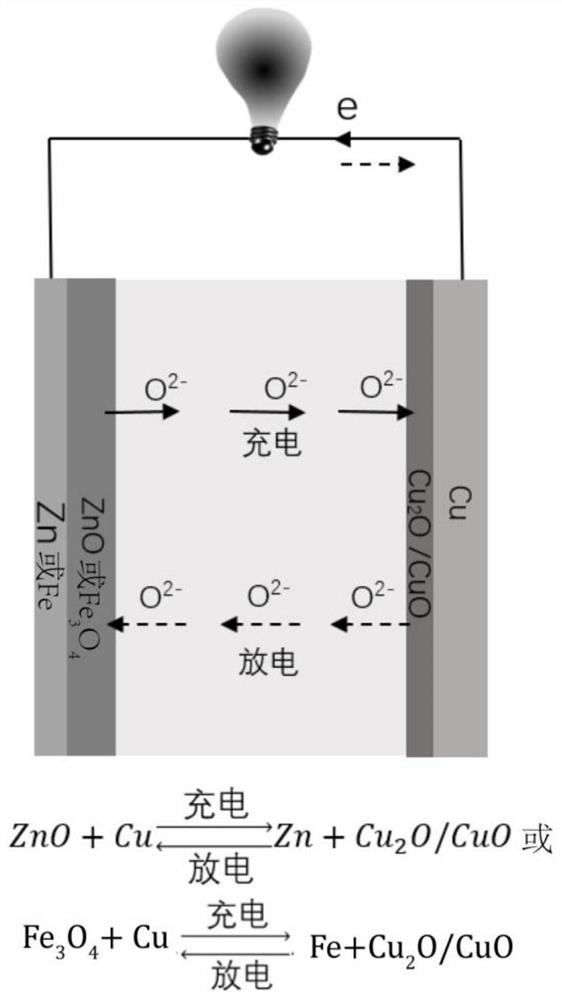
ActiveCN112952216BPrevent leakageEasy to store and transportFinal product manufactureSecondary cellsElectrical batteryOxygen ions
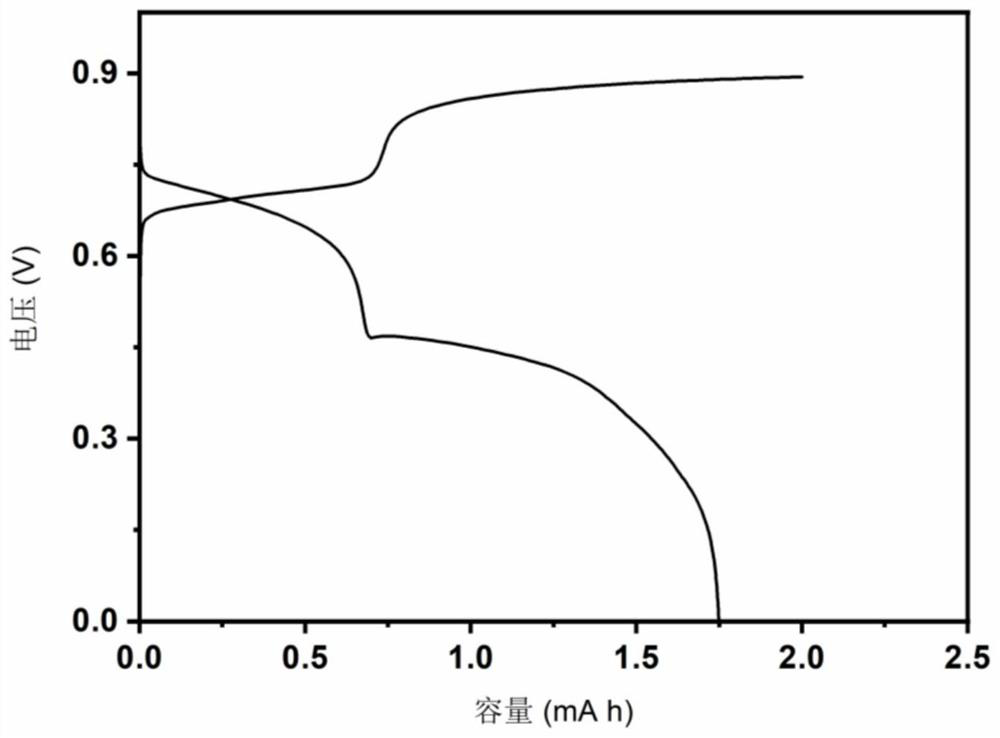
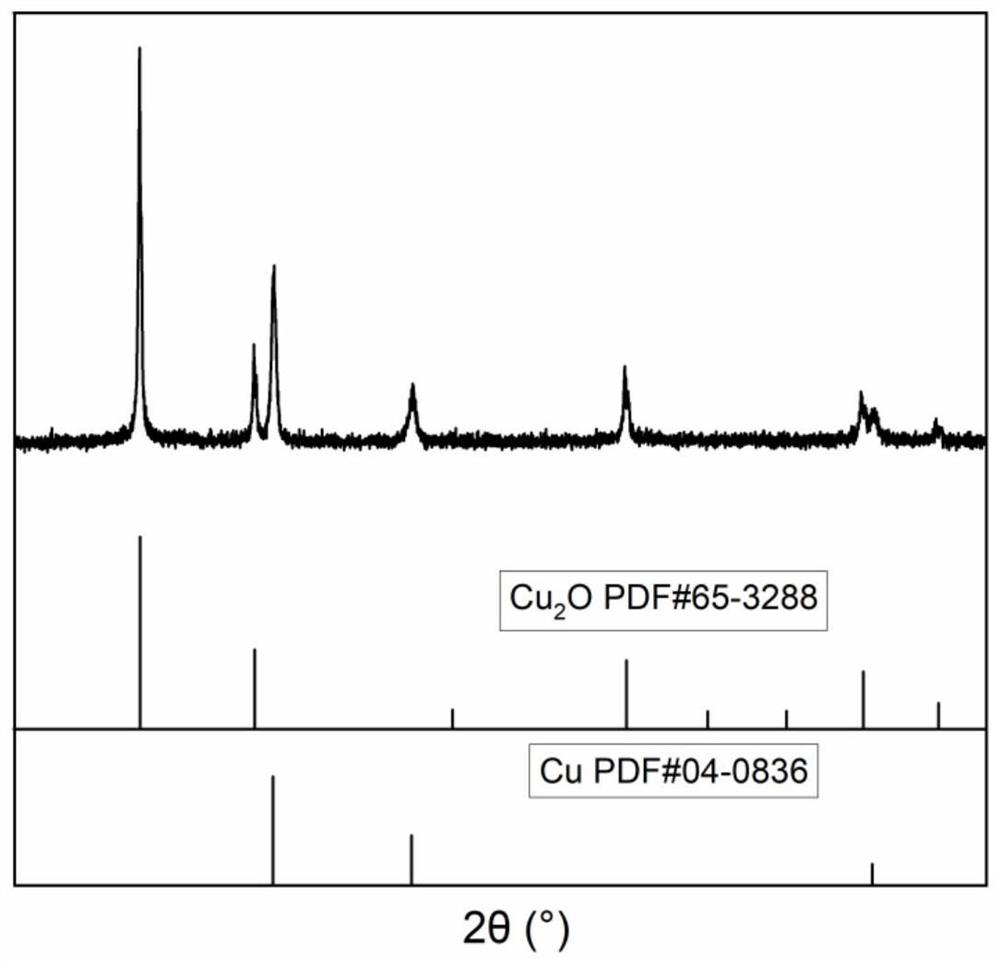
The invention is a new battery with oxygen ion conduction, which uses the oxide of a relatively inactive metal (such as copper, etc.) as the positive electrode, the active metal (such as iron, zinc, etc.) . During the discharge process, the oxygen ions in the metal oxide of the positive electrode enter the electrolyte, the positive electrode is reduced to a simple metal, the oxygen ions are transported to the negative electrode through the electrolyte, and form oxides with the negative metal; the charging process is the opposite. Therefore, the charge and discharge of the battery depends on the migration of oxygen ions in the electrolyte between the positive and negative electrodes to achieve charge and discharge, and the difference in the binding energy between the positive and negative metals and oxygen stores electrical energy. The metal materials and electrolytes of the battery are rich in sources and cheap, the battery manufacturing process is simple, and the metal electrodes are convenient to recycle, which is suitable for large-scale energy storage.
Owner:NANJING UNIV
Nonaqueous electrolyte secondary battery
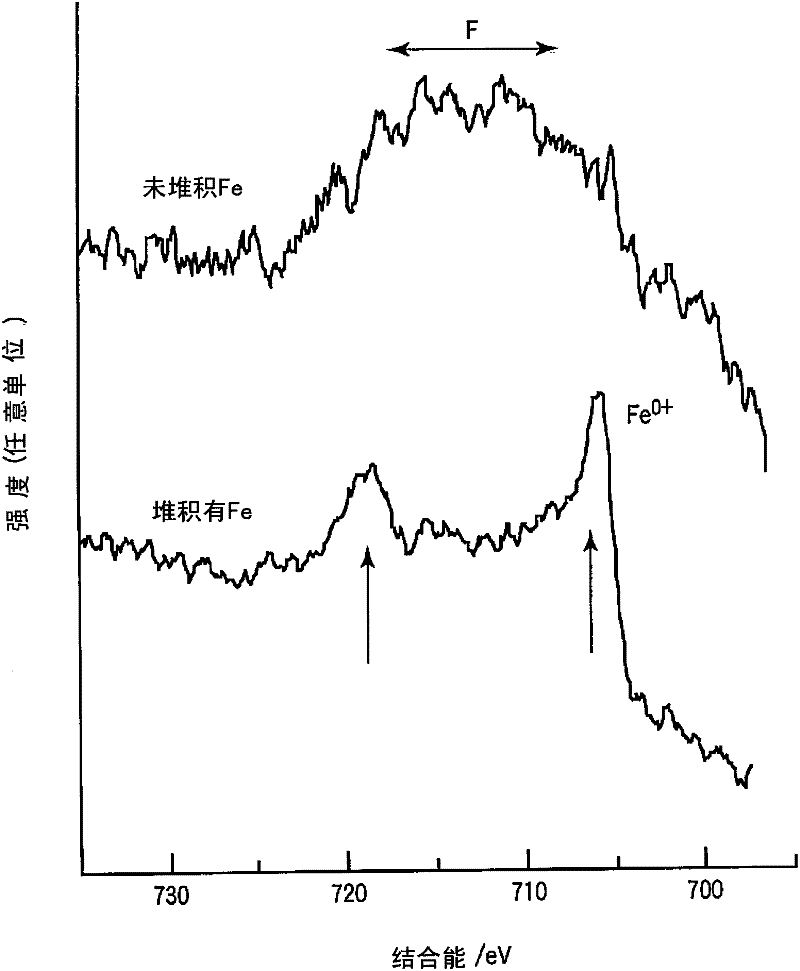
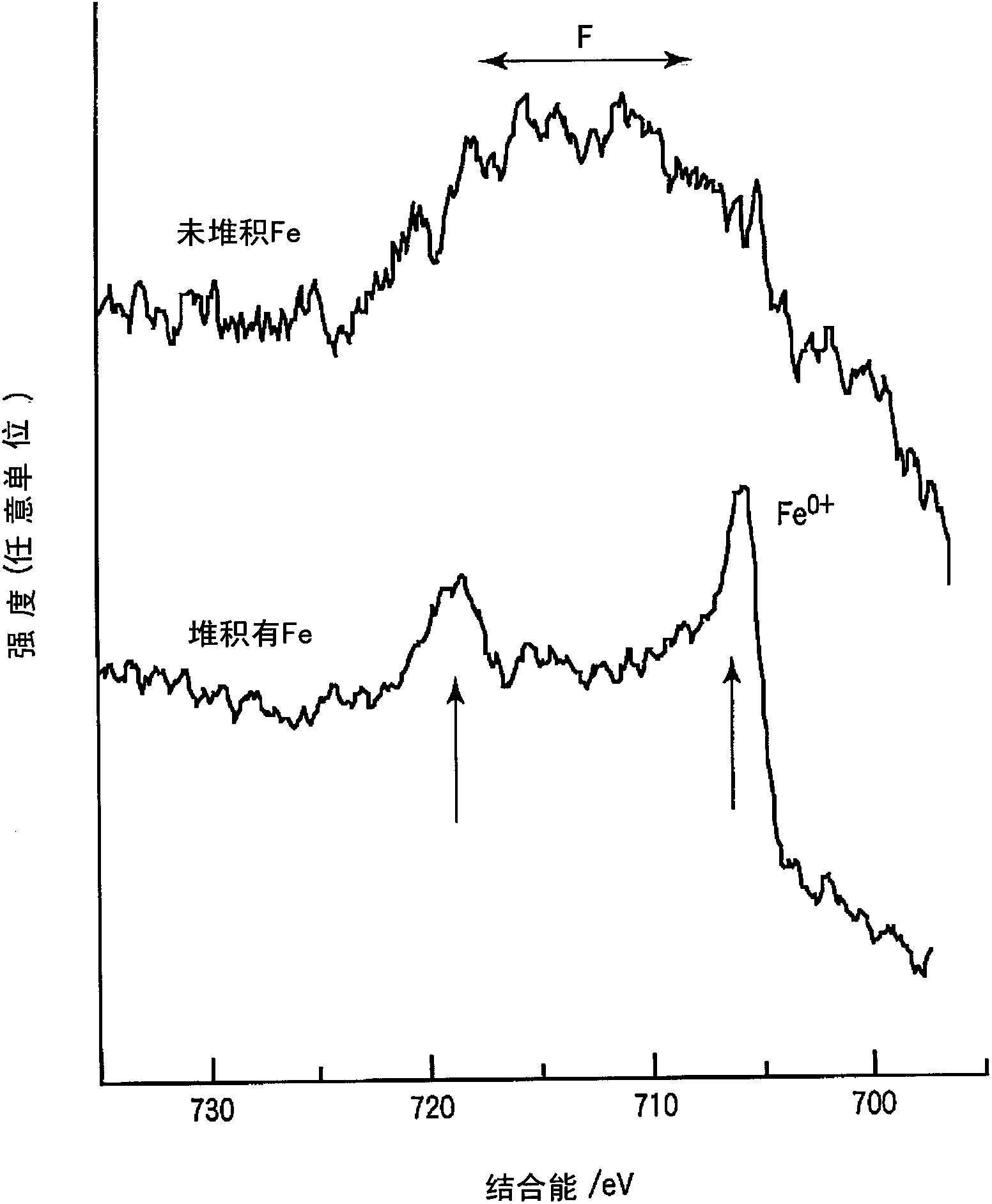
ActiveCN102414873AInhibition of self-dischargeFast charging performanceNegative electrodesSecondary cellsHigh rateIron metal
The invention relates to a nonaqueous electrolyte secondary battery comprising a negative electrode layer containing an active material (such as a titanium complex oxide) having a potential of not less than 0.5 V but not more than 2 V based on lithium metal at the time when lithium is inserted / removed. Since an SEI is rarely formed on the surface of a negative electrode which contains lithium-titanium complex oxide as a negative electrode active material, a side reaction is likely to occur between the negative electrode active material and the nonaqueous electrolyte solution, thereby easily causing self-discharge. Meanwhile, since 10-80% of each unit area of a negative electrode layer is composed of iron metal, the nonaqueous electrolyte secondary battery of the present invention is reduced in the area wherein the negative electrode layer surface and the nonaqueous electrolyte solution are in direct contact with each other. As a result, self-discharge is significantly suppressed, while maintaining discharge characteristics and high-rate charging performance with a large current.
Owner:KK TOSHIBA
Non-aqueous electrolyte secondary battery
ActiveCN102414873BInhibition of self-dischargeFast charging performanceSecondary cellsNegative electrodesMetallic lithiumMetal
The present invention relates to a non-aqueous electrolyte secondary battery comprising a negative electrode layer containing an active material (such as a titanium composite oxide) with a potential of 0.5 V to 2 V based on the metal at the time of intercalation / deintercalation of lithium. ). The negative electrode containing lithium-titanium composite oxide as the negative electrode active material hardly forms SEI on its surface, so side reactions between the negative electrode active material and the non-aqueous electrolyte are prone to occur, and self-discharge is likely to occur. The present invention can reduce the area of direct contact between the surface of the negative electrode layer and the non-aqueous electrolyte by forming 10% to 80% metallic iron per unit area on the above-mentioned negative electrode layer, and maintain the discharge characteristics and fast charging function during high current, and also Large self-discharge can be suppressed.
Owner:KK TOSHIBA
A kind of negative electrode for zinc-based flow battery and its battery and application
ActiveCN112928282BReduced Coulombic efficiencyAvoid direct contactCell electrodesRegenerative fuel cellsElectrical batteryConductive materials
The invention provides a long-life zinc-based liquid flow battery. The negative electrode of the battery includes a three-dimensional conductive carbon material and an inert conductive material; the thickness of the inert conductive material is 20%-50% of the three-dimensional conductive carbon material. The negative electrode and the battery of the present invention can effectively inhibit the self-discharge reaction of the positive electrode active material of the battery and the negative electrode active material in contact, improve the coulombic efficiency of the battery, and induce the reaction interface of zinc deposition to occur inside the electrode, thereby improving the coulombic efficiency of the zinc-based flow battery. efficiency.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Separator for battery
InactiveCN102725882BInhibition of self-dischargeMaintain capacityCell component detailsPolymer sciencePolyolefin
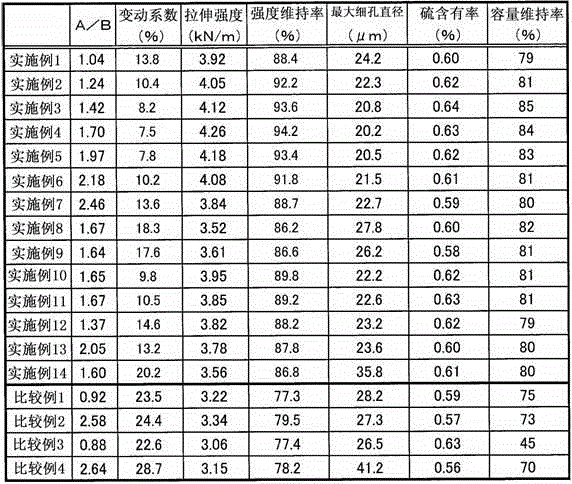
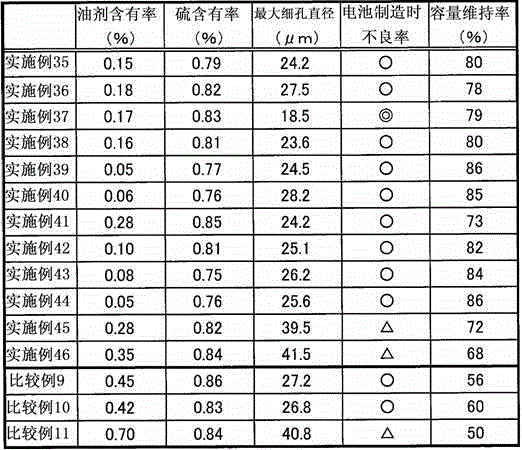
A separator for a battery which is produced by sulfonating a polyolefin nonwoven fabric that comprises core-sheath-type composite fibers each comprising polypropylene as a core component and high-density polyethylene as a sheath component, and which is characterized in that, in a DSC curve obtained by the differential scanning calorimetry of the core-sheath-type fibers and the separator, the ratio of the melting peak area (A) derived from the high-density polyethylene and appearing on the lower melting point side to the melting peak area (B) derived from the polypropylene and appearing on the higher melting point side (i.e., A / B) is 1.00 to 2.50 inclusive; and a separator for a battery, which is produced by sulfonating a nonwoven fabric that comprises at least polyolefin fibers, and which is characterized in that the content of an oily material in the nonwoven fabric that is not sulfonated yet is 0.40 mass% or less.
Owner:MITSUBISHI PAPER MILLS LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com