Multibiotic agents and methods of using the same
A technology of biological agents and vitamins, applied in anti-inflammatory agents, drug combinations, pharmaceutical formulations, etc., can solve the problem of underutilization of small molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
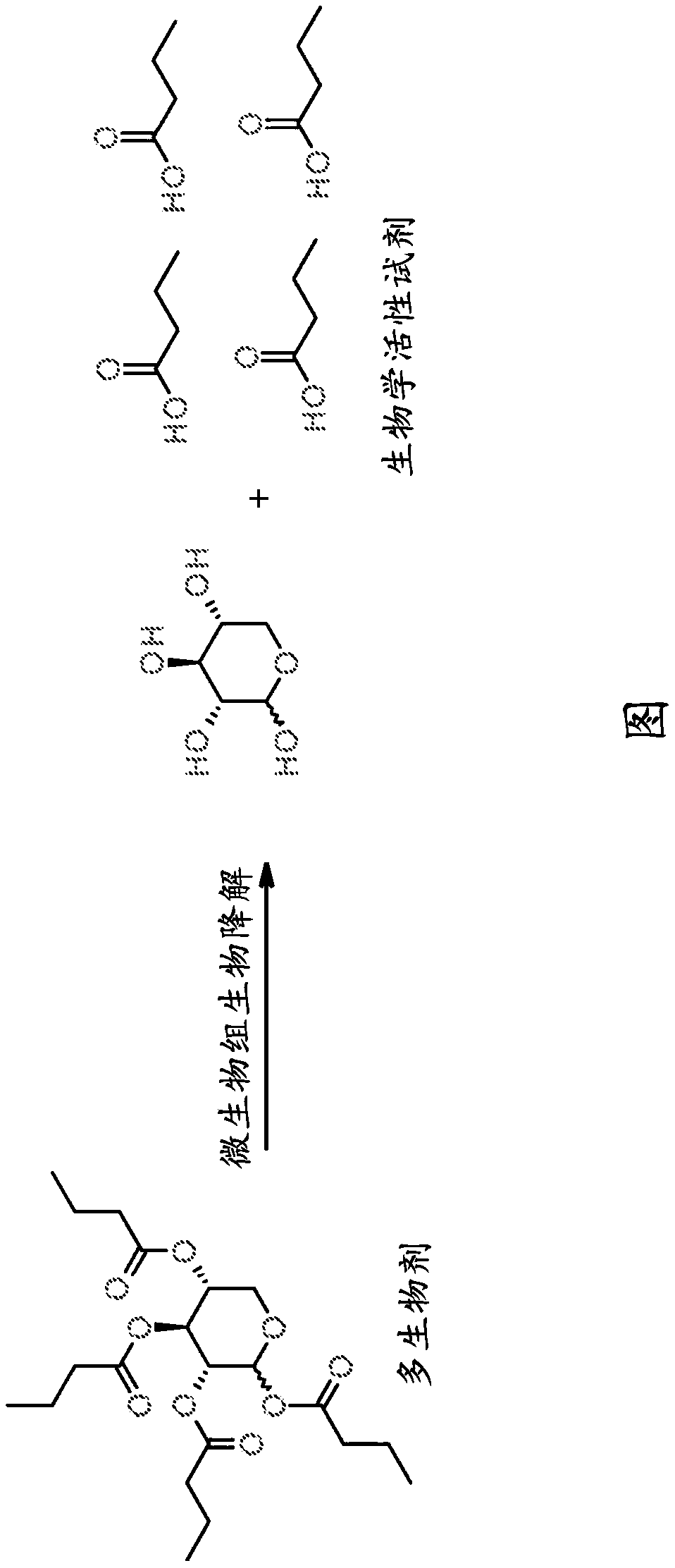
Image
Examples
preparation example Construction
[0388]Preparation of ester-containing multibiological agent
[0389] Ester-containing multibiotics can be prepared using commercially available starting materials, known intermediates, or by using synthetic methods known in the art (e.g., Wuts, P., Greene's Protective Groups in Organic Synthesis [Green's Protective Groups in Organic Synthesis]. ], 5th ed., method described in Wiley [Wiley Press] (2014).
[0390] The following exemplified schemes illustrate methods for preparing the multi-biological agent esters of the present invention. These methods are not limited to the production of the compounds shown, but can also be used to prepare various molecules such as the esters described herein. The compounds of the present invention can also be synthesized by methods not explicitly described in the schemes but within the skill of the art. Compounds can be prepared using readily available materials or known intermediates.
[0391] In the following schemes, Ar is optionally sub...
example 1
[0613] Example 1: [4-[(1E,6E)-7-[4-[2-(1H-indol-3-yl)acetyl]oxy-3-methoxy-phenyl]-3,5 -Dioxo-1,6-dienyl]-2-methoxy-phenyl]2-(1H-indol-3-yl)acetate
[0614] p-curcumin (3g, 8.14mmol, 1 equivalent), 2-(1H-indol-3-yl) acetic acid (7.13g, 40.72mmol, 5 equivalents), EDCI (7.49g, 39.09mmol, 4.8 equivalents) and 4 - A mixture of dimethylaminopyridine (4.78 g, 39.09 mmol, 4.8 equiv) in THF (100 mL) was degassed and washed with N 2 Purge 3 times, and then place the mixture under N 2 Stir at 15 °C for 3 h under atmosphere. The reaction mixture was mixed with brine (100 mL) and extracted with EtOAc (100 mL 3). The organic layer was washed with anhydrous Na 2 SO 4 Dry, filter and concentrate in vacuo. The residue was purified by column (petroleum ether:ethyl acetate=10:1 to 3:1), and concentrated to get crude product. The crude product was further purified by recrystallization from EtOAc (20 mL) to give pure product. (638mg, 935mmol, yield 11%, purity 96.39%) LC / MS: (M+H + ):683....
example 2
[0616] Example 2: 5-Amino-2-butyryloxy-benzoic acid
[0617] step 1:
[0618] at 15°C, N 2 To a mixture of 5-amino-2-hydroxy-benzoic acid (3 g, 19.59 mmol, 1 equiv) in methanol (50 mL) was added Boc in one portion under 2 O (4.28 g, 19.59 mmol, 4.50 mL, 1 equiv). The mixture was stirred at 15 °C for 5 h. The residue was poured into water (100 mL). The aqueous phase was extracted with EtOAc (100 mL), and the organic phase was washed with anhydrous Na 2 SO 4 Dry, filter and concentrate in vacuo. The residue was used in the next step without further purification. 5-(tert-butoxycarbonylamino)-2-hydroxy-benzoic acid (4 g, crude) was obtained as crude product.
[0619] Step 2:
[0620] Add 5-(tert-butoxycarbonylamino)-2-hydroxy-benzoic acid (4 g, 15.79 mmol, 1 equiv) and triethylamine (119.87 mg, 1.18 mmol, 164.88 μL, 1 equiv) at 0 °C in To a solution in THF (30 mL) was added butyryl chloride (126.22 mg, 1.18 mmol, 123.74 μL, 1 equiv) dropwise while maintaining the tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com