Benzoxazole derivative, and preparation method and application thereof
A technology for benzoxazole and derivatives, applied in the field of benzoxazole derivatives and their preparation, can solve the problems of poor solubility, difficult synthesis and purification, low oral bioavailability, etc., and achieves good inhibitory activity and good resistance. Inflammatory activity, good activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
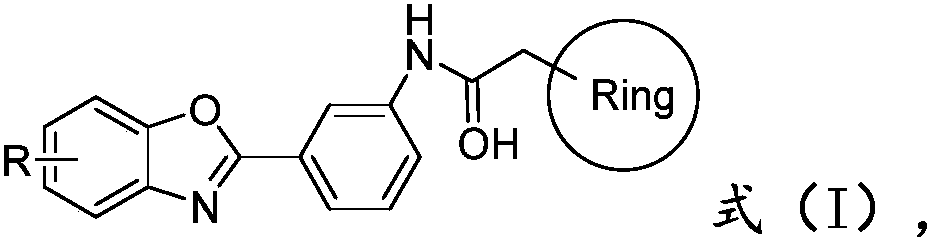
[0017] The present invention also provides a preparation method of a benzoxazole derivative having a structure shown in formula (I), comprising:
[0018] The compound of formula (II) structure and the compound of formula (III) structure are reacted, obtain the compound of formula (I) structure;
[0019]
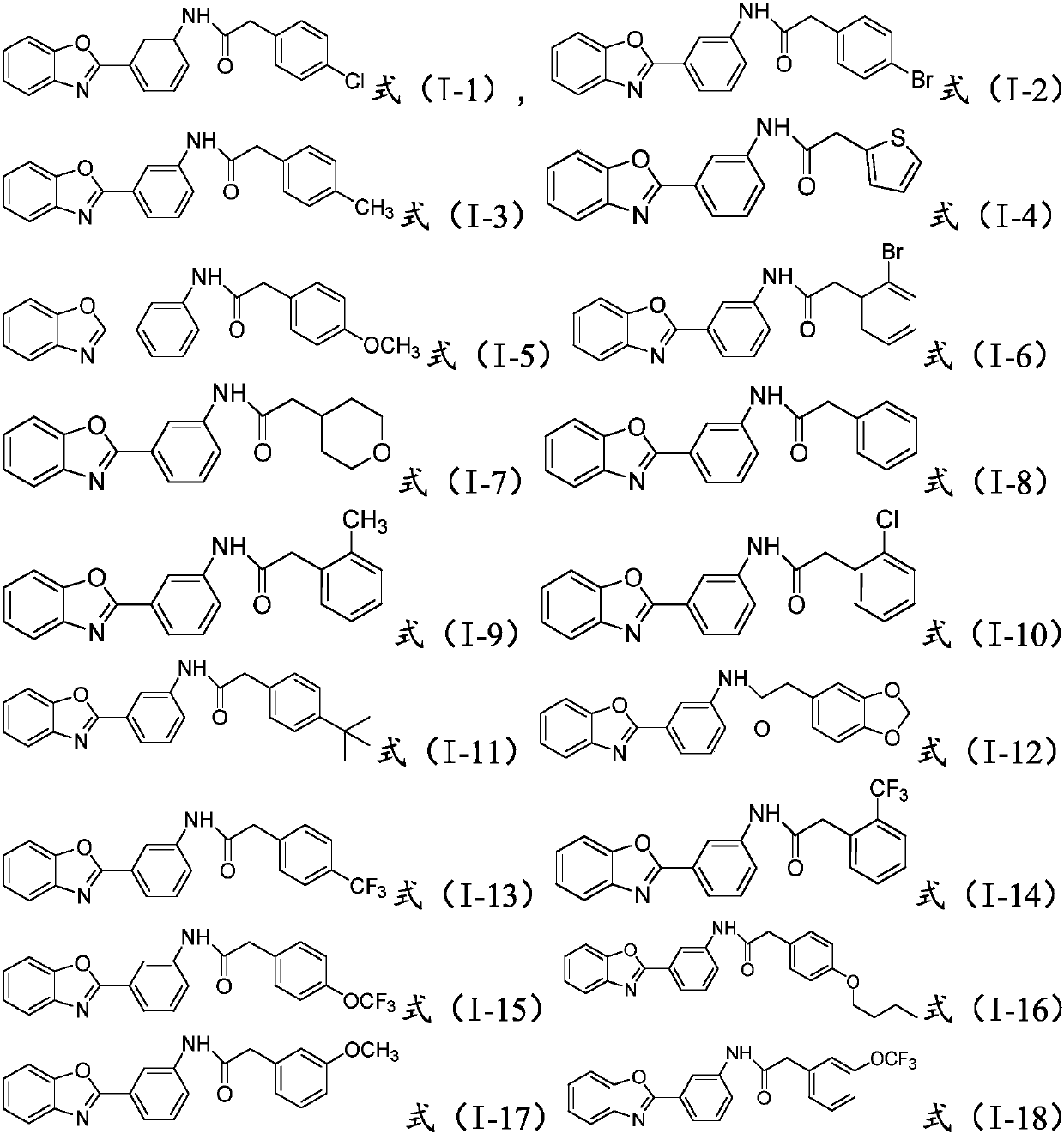
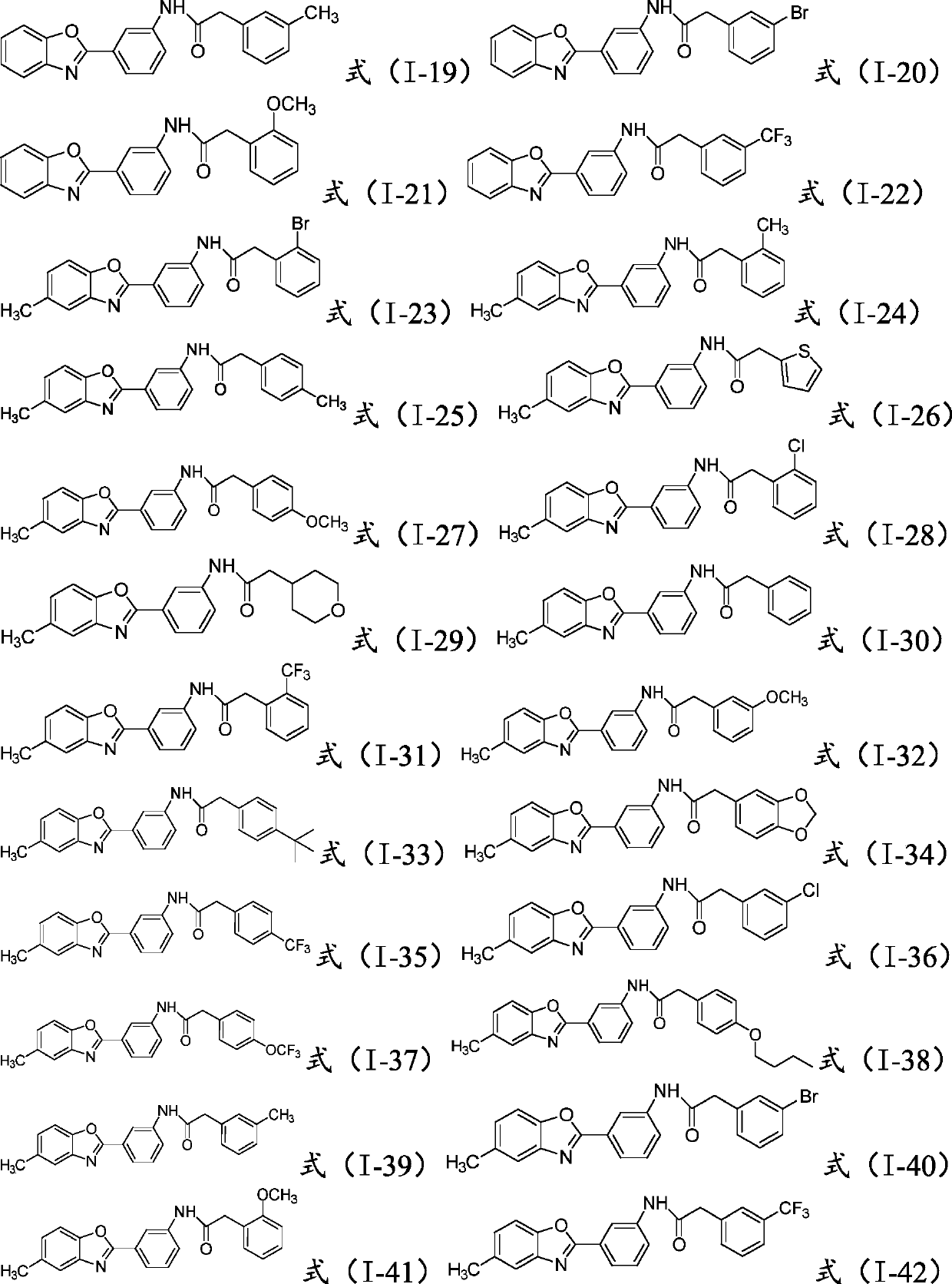
[0020] Wherein, the R is hydrogen, C1-C10 alkyl or halogen;
[0021] said It is a substituted or unsubstituted C4-C10 heterocycloalkyl group, a substituted or unsubstituted C4-C10 heterocyclic aryl group, or a substituted or unsubstituted C6-C20 aryl group.
[0022] According to the present invention, the present invention will react the compound of formula (II) structure and the compound of formula (III) structure, obtain the compound of formula (I) structure; Wherein, the present invention does not have special requirement to the method condition of reaction, the skilled artisan A skilled person can select a suitable preparation process according to the reaction raw m...
Embodiment 0
[0030]
[0031] Synthesis of 3-(benzo[d]oxazol-2-yl)aniline:
[0032] Add o-aminophenol (1g), m-aminobenzoic acid (1.256g), and 10mL polyphosphoric acid (PPA) into a 50mL round-bottomed flask, heat at 185°C, and reflux for 6h. After the reaction, cool to room temperature and use a cold Neutralize with 6N NaOH, filter the precipitate, and dry it. Dissolve the precipitate with ethyl acetate, filter several times, collect the filtrate, and spin dry. The product was passed through the column with petroleum ether:ethyl acetate (PE:EA) = 4:1, and the red solid was obtained as the product.
[0033] 1H NMR (600MHz, cdcl3) δ7.76-7.74 (m, 1H), 7.63 (d, J = 7.7Hz, 1H), 7.58-7.54 (m, 2H), 7.34-7.31 (m, 2H), 7.28 ( t, J=7.8Hz, 1H), 6.82(dd, J=8.0, 2.1Hz, 1H).
Embodiment 1
[0035]
[0036] Synthesis of N-(3-(benzo[d]oxazol-2-yl)phenyl)-2-(4-chlorophenyl)acetamide:
[0037] 3-(Benzo[d]oxazol-2-yl)aniline, 4-chlorophenylacetic acid, EDCI, HOBt, DIPEA were added to 10 mL of DMF in a molar ratio of 1:1:1.5:1.5:3, The reaction was monitored by TLC. After the reaction was completed, it was quenched by adding water, extracted, dried, and spin-dried. Add a small amount of ethyl acetate to precipitate the product, filter and dry.
[0038] 1 H NMR (600MHz, DMSO) δ8.58(s, 1H), 7.86(d, J=7.6Hz, 1H), 7.79-7.72(m, 3H), 7.52(t, J=7.9Hz, 1H), 7.43 -7.34(m, 6H), 3.68(s, 2H).
[0039] 13 C NMR(151MHz,DMSO)δ169.20,162.12,150.20,141.44,139.91,134.73,131.37,131.09,129.90,128.27,126.85,125.62,124.94,122.25,122.06,119.86,117.63,110.94,42.48,39.94,39.80 , 39.66, 39.52, 39.38, 39.24, 39.10.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com