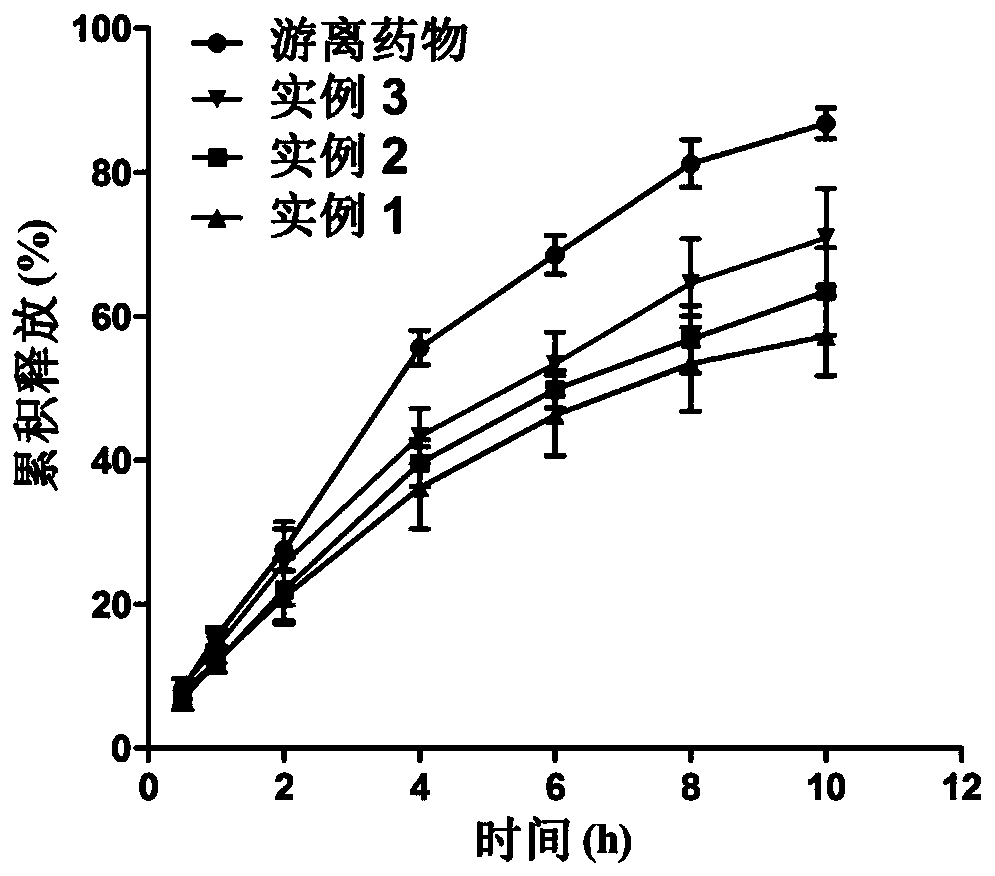
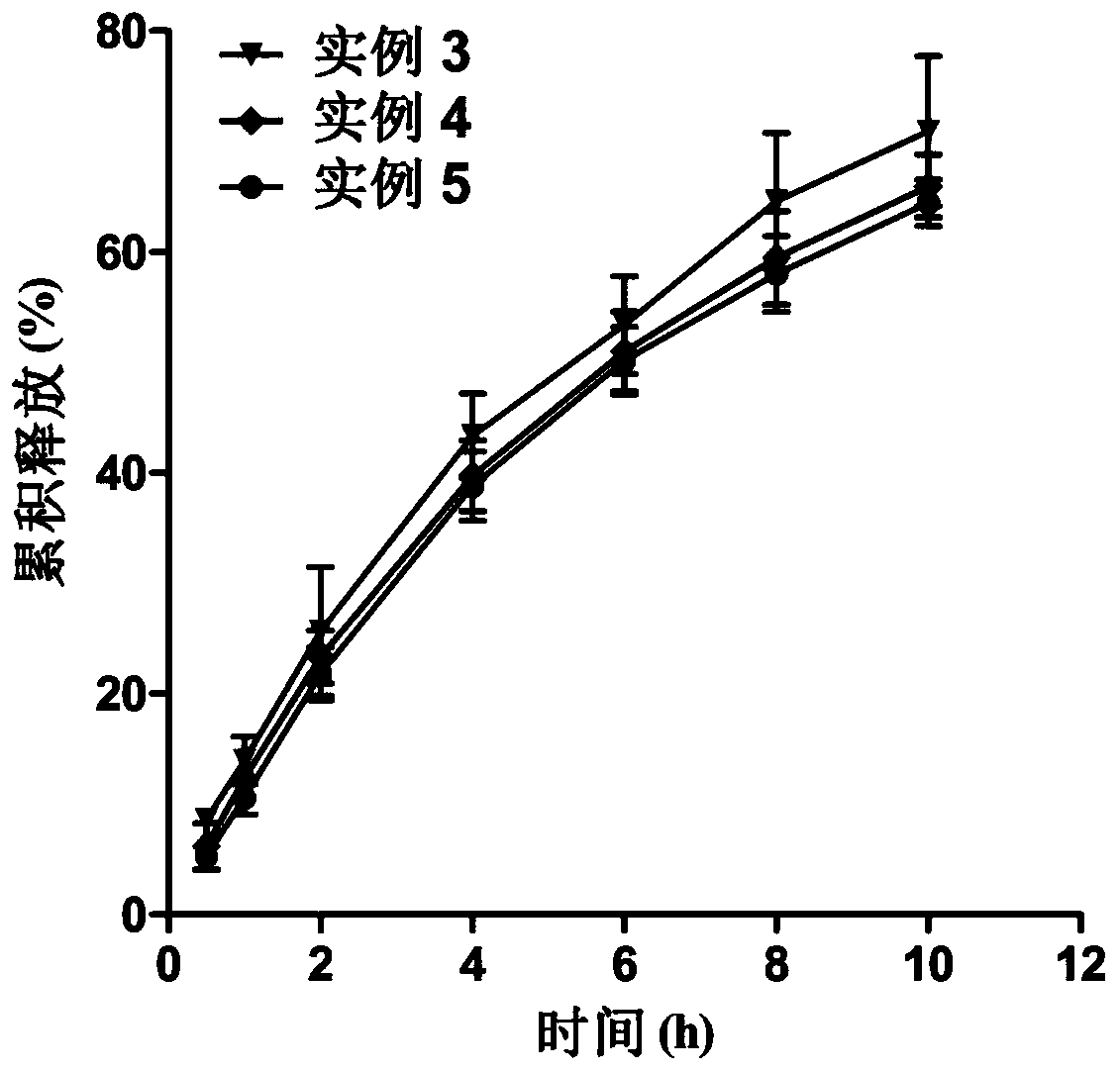
Etomidate nano preparation and preparation method thereof
A technology of etomidate and nano-preparation, applied in nanotechnology, nanotechnology, nanomedicine and other directions, can solve the problems of unusable and unfavorable preservation of nutrients, and achieve the effects of easy storage, non-irritant allergy and high safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Nano formulation of etomidate of the present invention
[0033] (1) Weigh 100mg lecithin and 10mg etomidate into 2 3ml test tubes, numbered a and b.
[0034] (2) Add 1ml of chloroform to the two test tubes a and b respectively, shake well and shake well until no solid particles remain on the tube wall.
[0035] (3) Inject all the solutions in a and b2 test tubes into a rotary evaporator at 25°C and maintain a constant speed of 60r / min to remove volatile solvents so that liposomes form a lipid dry film on the spherical wall.
[0036] (4) After the film is formed, continue to bubbling in nitrogen for 10 minutes to remove residual volatile organic solvents.
[0037] (5) Add 5 ml of phosphate buffered saline (PBS) with a pH of 7.35, shake and shake well until there are no solid particles remaining on the tube wall. After it is completely dissolved, a phosphate buffer solution with a concentration of 2 mg etomidate per ml is formed, and then the mixed solution is poured int...
Embodiment 2
[0039] Example 2 Nano formulation of etomidate of the present invention
[0040] (1) Weigh 90 mg of lecithin, 10 mg of pluronic P123, and 10 mg of etomidate into three 3 ml test tubes, numbered a, b, and c.
[0041] (2) Add 1ml of chloroform to the 3 test tubes of a, b, and c, shake well, and shake well until there are no solid particles on the tube wall.
[0042] (3) Inject all the solutions in the 3 test tubes of a, b, and c into the rotary evaporator at 25°C and maintain a constant speed of 60r / min to remove volatile solvents so that the liposomes form a lipid dry film on the spherical wall.
[0043] (4) After the film is formed, continue to bubbling in nitrogen for 10 minutes to remove residual volatile organic solvents.
[0044] (5) Add 5 ml of pH 7.35 phosphate buffered saline (PBS), shake and shake well until there are no solid particles remaining on the tube wall. When completely dissolved, a phosphate buffer solution with a concentration of 2 mg etomidate per ml is formed, and...
Embodiment 3
[0046] Example 3 Nano formulation of etomidate of the present invention
[0047] (1) Weigh 80mg lecithin, 20mg pluronic P123, and 10mg etomidate into 3 3ml test tubes, numbered a, b, c
[0048] (2) Add 1ml of chloroform to the 3 test tubes of a, b, and c, shake well, and shake well until there are no solid particles on the tube wall.
[0049] (3) Inject all the solutions in the 3 test tubes of a, b, and c into the rotary evaporator at 25°C and maintain a constant speed of 60r / min to remove volatile solvents so that the liposomes form a lipid dry film on the spherical wall.
[0050] (4) After the film is formed, continue to bubbling in nitrogen for 10 minutes to remove residual volatile organic solvents.
[0051] (5) Add 5 ml of phosphate buffered saline (PBS) with a pH of 7.35, shake and shake well until there are no solid particles remaining on the tube wall. When completely dissolved, a phosphate buffer solution with a concentration of 2 mg etomidate per ml is formed, and then the mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com