Preparation method of W-SSZ-13 zeolite with high crystallinity and high hydrophobicity
A technology of W-SSZ-13 and high hydrophobicity, applied in the field of preparation of W-SSZ-13 zeolite, can solve problems such as difficulty in synthesizing heteroatom zeolite, and achieve the effects of reducing synthesis cost, shortening crystallization time, and lowering reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
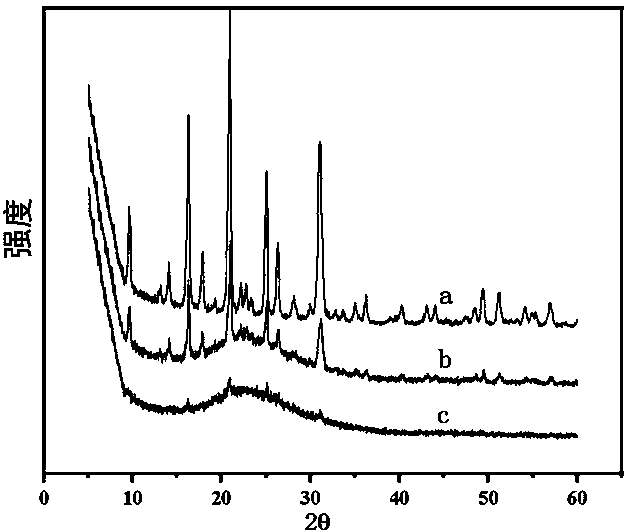
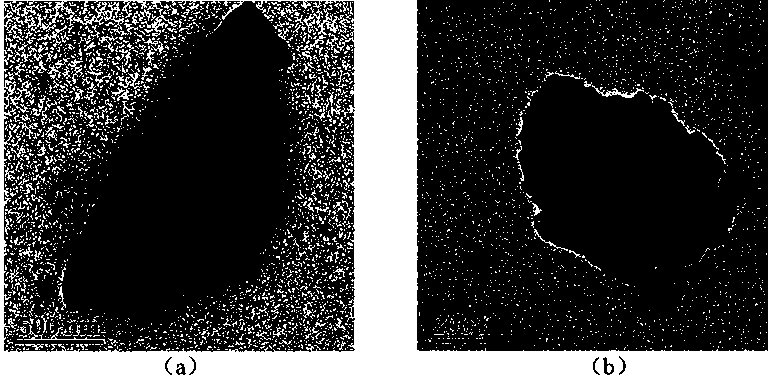
[0024] Example 1: Synthesis of fully crystalline SSZ-13
[0025] First, dissolve 0.22g of sodium metaaluminate in 19.7mL of water, stir for 15min to make the solution clear, weigh 0.12g of sodium hydroxide into the solution, stir for 5min, measure 4.8mL of N, N-trimethyl 1-adamantane aqueous solution of ammonium hydroxide (25%), stirring for 15 min. Finally, 3.5 mL of silica sol (30%) was measured and stirred for 4 hours. The aging solution was put into the reaction kettle and reacted hydrothermally at 160°C for 4 days. The sample was centrifuged and washed with water and dried at 110°C for 12 hours.
Embodiment 2
[0026] Example 2: Synthesis of incompletely crystalline SSZ-13 zeolite
[0027] First, dissolve 0.22g sodium metaaluminate in 19.7mL water, stir for 15min to make the solution clear, weigh 0.12g sodium hydroxide into the solution, stir for 5min, measure 4.8mL N, N-trimethyl-1- Adamantane ammonium hydroxide aqueous solution (25%). Stir for 15min. Finally, measure 3.5 mL of silica sol (30%) and stir for 4 hours. The aging solution was put into the reaction kettle and reacted hydrothermally at 160°C for 1 day. The sample was centrifuged and washed with water and dried at 110°C for 12 hours.
Embodiment 3
[0028] Example 3: Synthesis of incompletely crystalline SSZ-13 zeolite doped with tungsten
[0029] First, dissolve 0.22g of sodium metaaluminate in 19.7mL of water, stir for 15min to make the solution clear, weigh 0.12g of sodium hydroxide into the solution, stir for 5min, measure 4.8mL of N, N-trimethyl-1 -Amantadine ammonium hydroxide aqueous solution (25%), stir for 15 min. Weigh 0.037g of sodium tungstate into the solution and stir for 15 minutes. Finally, 3.5 mL of silica sol (30%) was measured and stirred for 4 hours. In the future, the aging solution will be put into the reaction kettle, and hydrothermally reacted at 160°C for 1 day. The sample was centrifuged and washed with water and dried at 110°C for 12 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com