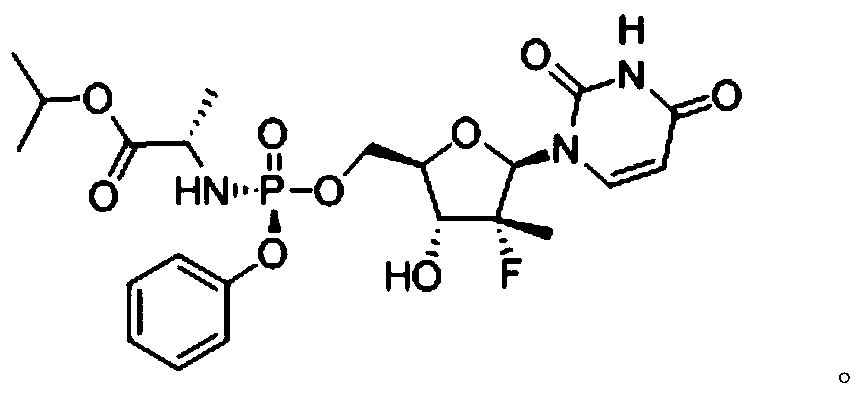
Method for preparing sofosbuvir intermediate by using microfluid reaction device
A technology for microfluidic reactions and intermediates, which is applied in the field of preparation of sofosbuvir intermediates, can solve the problems of "three wastes" pollution, high production cost, large refrigeration energy consumption, etc., and achieves improved reaction efficiency, stable reaction, and improved conversion rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
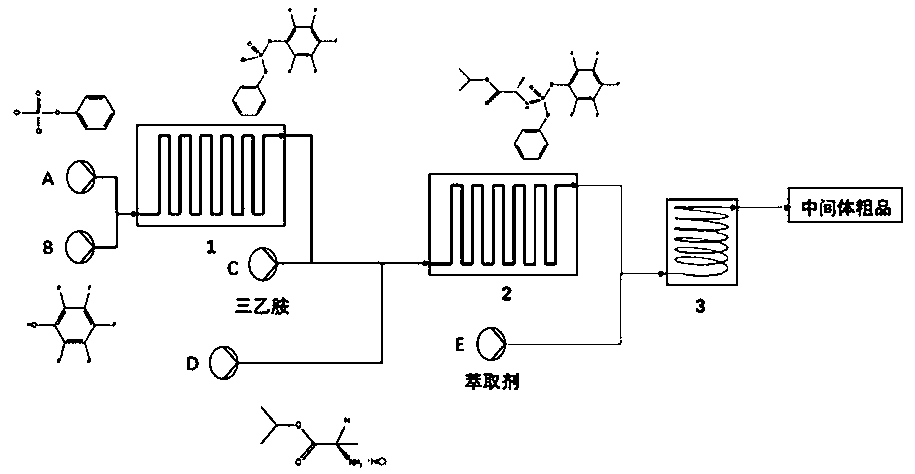
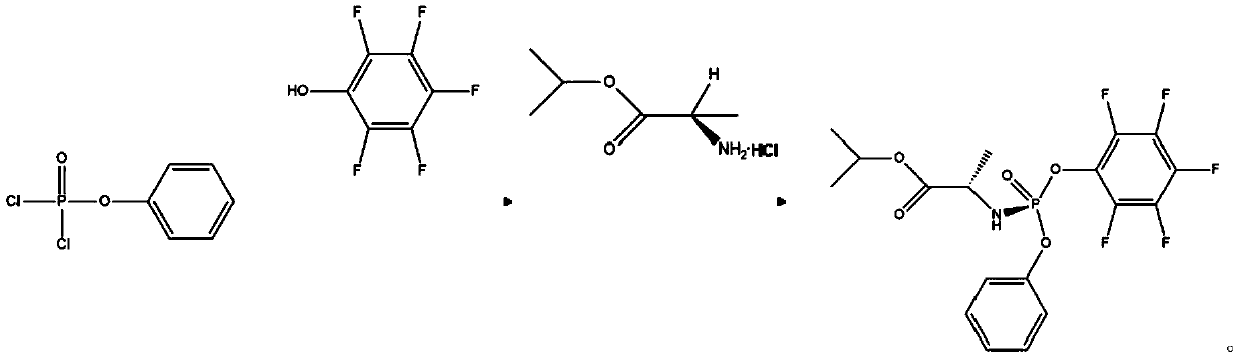
[0047] Dissolve 2.1g of phenyl dichlorophosphate in 25mL of chloroform to form reaction solution A, and then dissolve 1.84g of pentafluorophenol in 25mL of chloroform containing 1.01g of triethylamine to form reaction solution B. Liquids A and B were respectively pumped into the first microchannel reactor for mixed reaction. The flow rate of the metering pump was set to 0.5mL / min, the temperature of the microchannel reactor was set to 0°C, and the pressure was about 1.5Mpa. The reaction effluent and 2.02g of triethylamine is mixed and injected into the second microchannel reactor, the flow rate of the metering pump is set to 0.5mL / min, 1.68g of L-alanine isopropyl hydrochloride is dissolved in 50mL of chloroform, and the reaction Liquid D, the reaction solution D is pumped into the second microchannel reactor, and the flow rate of the metering pump is set to 0.5mL / min, and reacts with the mixed solution of the reaction effluent and triethylamine in the second microchannel react...
Embodiment 2
[0050] Dissolve 2.1g of phenyl dichlorophosphate in 25mL of chloroform to form reaction solution A, and then dissolve 1.84g of pentafluorophenol in 25mL of chloroform containing 1.01g of triethylamine to form reaction solution B. Liquids A and B were respectively pumped into the first microchannel reactor for mixed reaction. The flow rate of the metering pump was set at 1mL / min, the temperature of the microchannel reactor was set at 20°C, and the pressure was about 1.5Mpa. After the mixed flow of g triethylamine is injected into the second microchannel reactor, the flow rate of the metering pump is set to be 1mL / min, and 1.68g of L-alanine isopropyl hydrochloride is dissolved in 50mL of chloroform to configure the reaction solution D , the reaction solution D is pumped into the second microchannel reactor, the flow rate of the metering pump is set to 1mL / min, and reacts with the mixed solution of the reaction effluent and triethylamine in the second microchannel reactor to obta...
Embodiment 3
[0053] Dissolve 2.1g of phenyl dichlorophosphate in 15mL of chloroform to form reaction solution A, and then dissolve 1.84g of pentafluorophenol in 15mL of chloroform containing 1.01g of triethylamine to form reaction solution B. Liquids A and B were respectively pumped into the first microchannel reactor for mixed reaction. The flow rate of the metering pump was set to 0.5mL / min, the temperature of the microchannel reactor was set to 0°C, and the pressure was about 1.5Mpa. The reaction effluent and Mix 2.02g of triethylamine and inject it into the second microchannel reactor, set the flow rate of the metering pump to 0.5mL / min, dissolve 1.68g of L-alanine isopropyl hydrochloride in 50mL of chloroform, and configure it as a reaction solution D, the reaction solution D is pumped into the second microchannel reactor, the flow rate of the metering pump is set to 0.5mL / min, and the mixed solution of the reaction effluent and triethylamine is reacted in the second microchannel react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com