Copper-doped quasi-two-dimensional full-inorganic perovskite material and preparation method thereof
A copper-doped, inorganic calcium technology, used in luminescent materials, chemical instruments and methods, nanotechnology for materials and surface science, etc., can solve problems such as poor conductivity of perovskite films, improve optical properties, increase Quantum yield, effect of reducing defect states
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1Cu 0.6 -CsPb 0.4 Cl 3 preparation of
[0064] (1) Add 0.4g Cs 2 CO 3 , 20mL of octadecene and 1.5mL of oleic acid were added to a three-necked flask, heated to 120°C under vacuum, and then passed through Ar to react for 1h to obtain a precursor;
[0065] (2) 0.08g CuCl 2 , 1mL oleylamine and 5mL octadecene were added to a three-necked flask, and heated to 100°C under the protection of Ar for later use;
[0066] (3) 0.08g CuCl 2 , 0.114g PbCl 2 and 10 mL of octadecene were added to a three-necked flask, heated to 120°C under vacuum, and 1 mL of oleic acid and 1 mL of oleylamine were added, followed by passing through Ar for 10 minutes and adding 1 mL of tri-n-octylphosphine (TOP), Then continue to react for 50min. Then the temperature was raised to 200°C, and 1 mL of the precursor prepared in step (1) was added immediately, reacted for 1 min, and then cooled to room temperature with ice water;
[0067] (4) Cu-CsPbCl obtained in step (3) 3 CuCl obtaine...
Embodiment 2
[0069] Example 2Cu 0.6 -CsPb 0.4( Cl / Br) 3 preparation of
[0070] (1) Add 0.4g Cs 2 CO 3 , 20mL of octadecene and 1.5mL of oleic acid were added to a three-necked flask, heated to 120°C under vacuum, and then passed through Ar to react for 1h to obtain a precursor;
[0071] (2) 0.08g CuCl 2 , 1mL oleylamine and 5mL octadecene were added to a three-necked flask, and heated to 100°C under the protection of Ar for later use;
[0072] (3) 0.08g CuCl 2 , 0.114g PbCl 2 and 10 mL of octadecene were added to a three-necked flask, heated to 120°C under vacuum, and 1 mL of oleic acid and 1 mL of oleylamine were added, followed by passing through Ar for 10 minutes and adding 1 mL of tri-n-octylphosphine (TOP), Then continue to react for 50min. Then the temperature was raised to 200°C, and 1 mL of the precursor prepared in step (1) was added immediately, reacted for 1 min, and then cooled to room temperature with ice water;
[0073] (4) Cu-CsPbCl obtained in step (3) 3 CuCl ob...
experiment example 1
[0076] Experimental example 1 Structural characterization of copper-doped all-inorganic perovskite materials
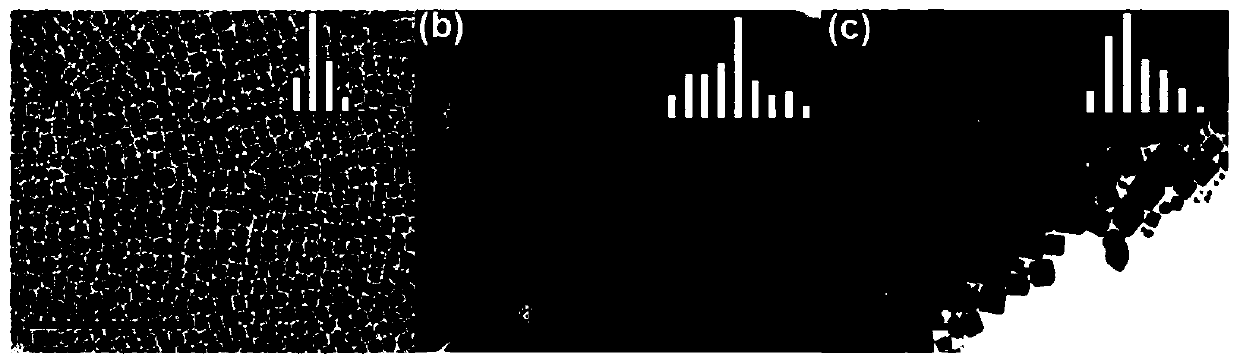
[0077] Adopt FEI Tecnai G2 F20 S-TWI type TEM to the purple light Cu that prepares in embodiment 1 and embodiment 2 0.6 -CsPb 0.4 Cl 3 and Blu-ray Cu 0.6 -CsPb 0.4 (Br / Cl) 3 The morphology and two-dimensional size were characterized, and the results are as follows figure 1 shown.
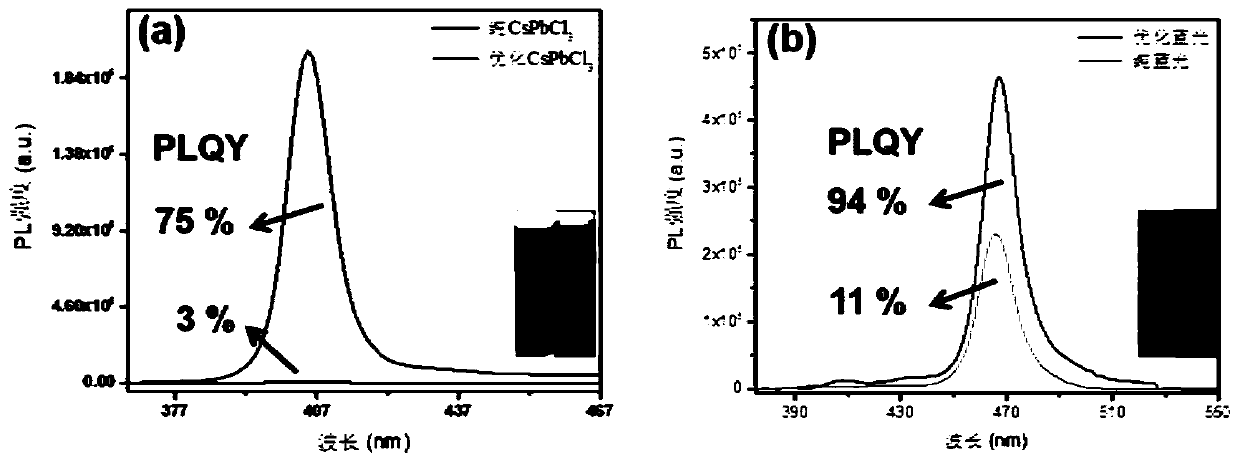
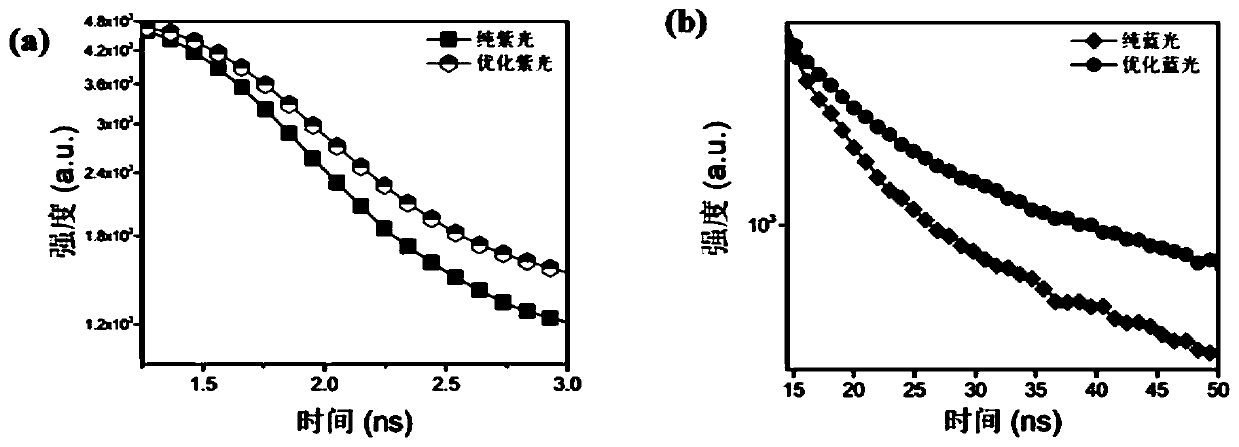
[0078] from figure 1 It can be seen from figure 1 Middle (a) undoped CsPbCl 3 It still has the traditional quantum dot morphology, with an average particle size of 7.7nm. And the optimized Cu-CsPbCl prepared by the present invention after two-step passivation 3 Then it presents the microscopic morphology of two-dimensional nanosheets, with an average particle size of 47.5nm. After anion exchange, although the prepared Cu-CsPb(Br / Cl) 3 The size of the blue-light nanosheets was slightly reduced (average particle size was 19.23 nm), but the morphology of the two-dimensional nanoshe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com