Novel plasma perfusion resin for treating nephropathy, and preparation method and application thereof
The technology of plasma perfusion and plasma perfusion device is applied in the field of novel plasma perfusion resin for the treatment of nephropathy and its preparation, which can solve the problems of complex modification method and poor applicability, and achieves improvement of blood compatibility, inhibition of bacterial function, and good industrial application. Foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
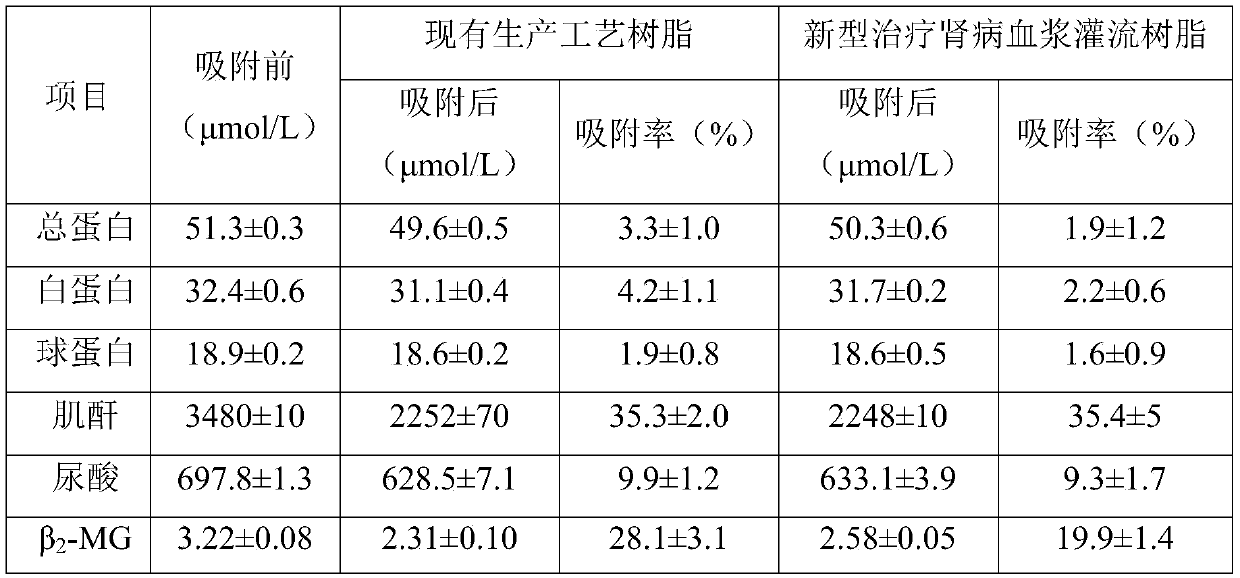
[0028] The polystyrene-divinylbenzene resin (model HB-H-10-1) was rinsed with absolute ethanol for 72 hours to remove organic impurities in the plasma perfusion resin for the treatment of renal disease, and then rinsed with water for injection until neutral ; Then rinse with 5wt% NaOH solution for 72h until the NaOH solution flowing out after rinsing is clear, no peculiar smell and no fine resin, and then rinse with water for injection until neutral; then rinse with 5wt% HCl Wash for 72 hours until the HCl is clear, odorless and free of finely divided resin after rinsing, and then rinse with water for injection until neutral; then treat for 20 minutes at a temperature of 121 ° C and a pressure of 103.4 kPa for high temperature and high pressure sterilization to obtain Existing production process resin;
[0029] In a sterile environment, after sealing the existing production process resin, sterilized preservation solution and human serum albumin, mix them in a shaker at 37°C an...
Embodiment 2
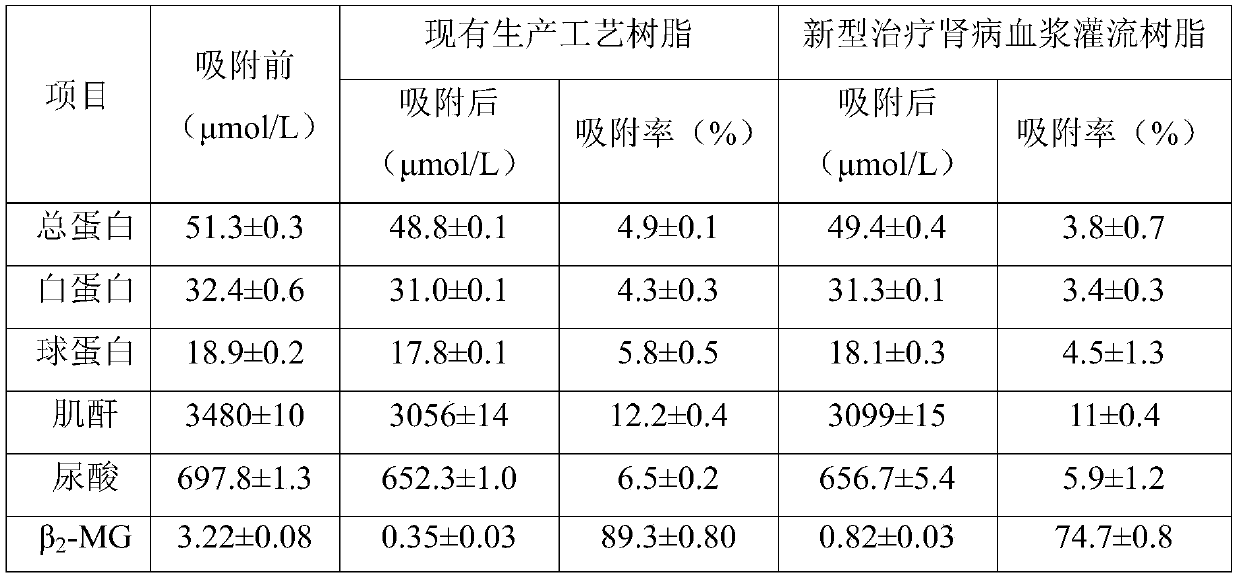
[0047] The polystyrene-divinylbenzene resin (model HB-H-10-2) was rinsed with absolute ethanol for 24 hours to remove organic impurities in the plasma perfusion resin for the treatment of renal disease, and then rinsed with water for injection until neutral ; Then rinse with 5wt% NaOH solution for 24h until the NaOH solution flowing out after rinsing is clear, no peculiar smell and no fine resin, and then rinse with water for injection until neutral; then rinse with 5wt% HCl Wash for 24 hours until the HCl is clear, no peculiar smell, and no finely divided resin after rinsing, and then rinse with water for injection until neutral; then treat for 15 minutes at a temperature of 121 ° C and a pressure of 103.4 kPa for high temperature and high pressure sterilization to obtain Existing production process resin;
[0048] In a sterile environment, after sealing the existing production process resin, sterilized preservation solution and human serum albumin, mix for 4 hours in a shake...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com