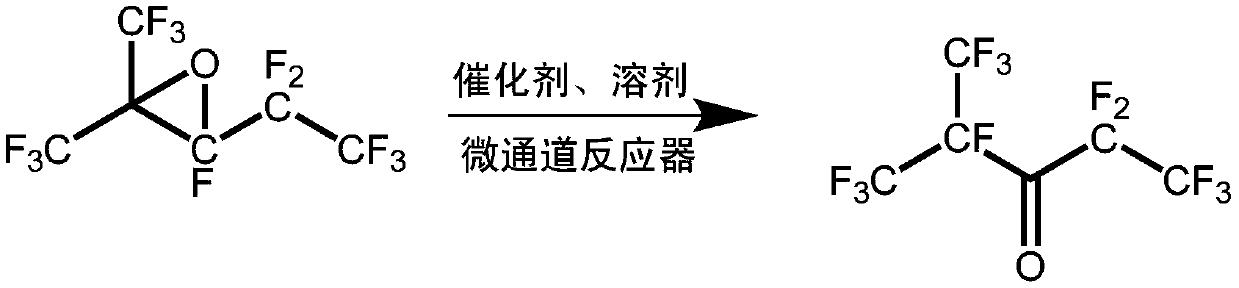
Method for synthesizing perfluoro(2-methyl-3-pentanone)
A technology for the synthesis of perfluorohexanone and its application in chemical instruments and methods, preparation of heterocyclic compounds, chemical/physical/physicochemical processes, etc., which can solve the problem of low formula conversion rate, easy deactivation of composite catalysts, and low reaction yield. Instability and other problems, to achieve the effects of less three wastes, easy automatic control and scale-up production, and shorten synthesis reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] This embodiment includes the following steps:
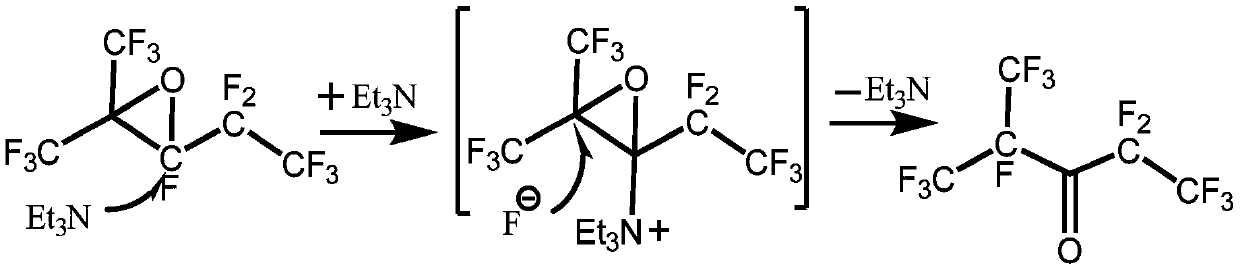
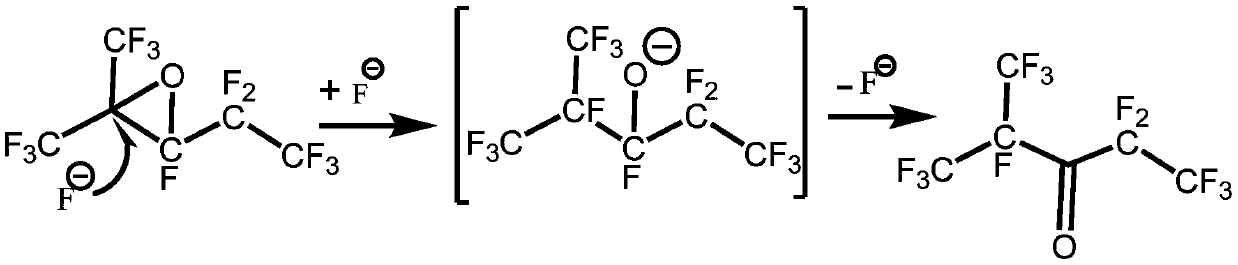
[0034] Step 1, isomerization synthesis reaction: Dissolve 0.01mol perfluoro-2,3-epoxy-2-methylpentane in 10mL acetonitrile to obtain perfluoro-2,3-epoxy-2-methylpentane solution, and then perfluoro-2,3-epoxy-2-methylpentane solution and catalyst triethylamine are sent into the microchannel reactor through a micro-injection pump, and then stay at a temperature of 10°C for 600s for isomerization Combination reaction to obtain reaction product; the amount of the substance of the catalyst triethylamine is 2.5% of the amount of the substance of perfluoro-2,3-epoxy-2-methylpentane, and the microchannel reactor of the microchannel reactor The channel aperture is 100μm and the depth is 40μm;
[0035] Step 2. Separation and purification: Cool the reaction product obtained in step 1 to 0°C to 5°C and then let it stand for stratification, then collect the lower layer after standing and stratification for rectification treatment, and...
Embodiment 2
[0042] The difference between the synthesis method of embodiment 2 and embodiment 3 and embodiment 1 is: the temperature of the isomerization synthesis reaction is different, the reaction temperature and the corresponding conversion rate and yield of embodiment 1 to embodiment 3 are shown in the following table 1 Show.
[0043] The temperature of reaction of table 1 embodiment 1~embodiment 3 and corresponding conversion rate and productive rate
[0044]
[0045] As can be seen from Table 1, the optimal reaction temperature for the synthesis of perfluorohexanone by the isomerization reaction of the present invention is 30° C., and when the reaction temperature is lower than 30° C., the degree of reaction progress is low, and the conversion rate and the productive rate are all low. When the reaction temperature exceeds 30°C, the by-products generated by the reaction begin to increase, and both the conversion rate and the yield begin to decrease. Therefore, the temperature of...
Embodiment 4~ Embodiment 10
[0047] Embodiment 4~Example 10 differs from the synthetic method of Example 2 in that: the type of catalyst used is different. The catalyst used in Example 4~Example 10 and Example 2 and the corresponding conversion rate and yield are as follows Table 2 shown.
[0048] The catalyst that table 2 embodiment 4~embodiment 10 and embodiment 2 adopts and corresponding conversion ratio and productive rate
[0049]
[0050] As can be seen from Table 2, various metal fluorides and organic amine catalysts have a certain catalytic effect on the isomerization reaction, and the catalytic effect of organic amine catalysts is better than that of metal fluorides, and among them triethylamine best catalytic effect. Therefore, the isomerization reaction catalyst is preferably triethylamine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com