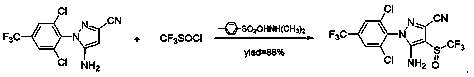
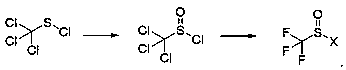
Preparation method of trifluoromethyl sulfinyl halide
A technology of trifluoromethyl sulfinyl halide and trichloromethyl sulfinyl chloride is applied in the preparation of sulfonic acid, the preparation of organic compounds, chemical instruments and methods, etc., and can solve the problems of compression filling, high toxicity, etc. To achieve the effect of saving manufacturing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: In a four-neck flask made of glass, add 186g of sulfuric acid, heat up to 60°C, 245.4g (3.0mol) of sodium perborate, replace with nitrogen, stir for 30 minutes, drop into another containing 186g of trichloromethylsulfur chloride (1.0mol) glass reaction vial, controlled in the sampling center, 6% trichloromethylsulfinyl chloride, 88.8% trichloromethylsulfinyl chloride, 5.2% trichloromethanesulfinyl chloride, dichloromethane extraction and removal of dichloromethane Chloromethane, a total of 205 g of the mixture was obtained, and 175.9 g of trichloromethylsulfinyl chloride was obtained after rectification under reduced pressure at 5 mmHg, with a content of 99.5% and a yield of 87.1%.
Embodiment 2
[0034] Example 2: In a four-neck flask made of glass, add 186g of sulfuric acid, cool down to 0°C, 81.8g (1.0mol) of sodium perborate, replace with nitrogen, stir for 30 minutes, drop into another containing 186g of trichloromethylsulfur chloride (1.0mol) glass reaction vial, controlled in the sampling center, 6% trichloromethylsulfinyl chloride, 93.9% trichloromethylsulfinyl chloride, 0.1% trichloromethanesulfinyl chloride, dichloromethane extraction and removal of dichloromethane Chloromethane, a total of 205g of the mixture was obtained, and 185.9g of trichloromethylsulfinyl chloride was obtained after rectification under reduced pressure at 5mmHg, with a content of 99.5% and a yield of 92.1%.
Embodiment 3
[0035] Example 3: In a four-necked glass flask, add 186g of sulfuric acid, heat up to 100°C, 81.8g (2.0mol) of sodium perborate, replace with nitrogen, stir for 30 minutes, drop into another containing 186g of trichloromethylsulfur chloride (1.0mol) glass reaction vial, sampling control, 0% trichloromethylsulfinyl chloride, 49.0% trichloromethanesulfinyl chloride, 51.0% trichloromethanesulfonyl chloride, use dichloromethane to remove dichloromethane Chloromethane, a total of 220 g of the mixture was obtained, and 100 g of trichloromethanesulfinyl chloride was obtained after rectification under reduced pressure at 5 mmHg, with a content of 99.5% and a yield of 49.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com