A kind of influenza A virus detection primer, probe and kit thereof
A type of influenza A virus and kit technology, applied in the field of nucleic acid amplification, can solve the problems of many false negatives, loss of clinical significance, long time-consuming virus isolation and culture, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1, the method for using the kit for detecting influenza virus
[0021] (1) Nucleic acid extraction: take clinical samples to be tested, and extract viral nucleic acid;
[0022] (2) Constant temperature amplification: Mix 15 μl constant temperature amplification buffer, 10 μl constant temperature amplification enzyme solution, and 25 μl clinical sample solution obtained in step (1) to form a 50 μl reaction solution, vortex and oscillate evenly, and inject the primer set and probe In the amplification reactor; place the reactor in a constant temperature amplification instrument, and react at 40°C for 50min;
[0023] (3) Result determination: After the reaction is over, determine whether the sample liquid to be tested contains the genome of the corresponding influenza virus according to the amplification curve of the sample in each reaction chamber; if the sample to be tested produces an S-type amplification curve, Then the sample to be tested contains the geno...
Embodiment 2
[0024] Embodiment 2, the detection of actual clinical sample
[0025] 1. Extraction of viral nucleic acid from clinical samples
[0026] Viral nucleic acid extraction from clinical samples was carried out using commercial kits, such as QIAamp Viral RNA Mini Kit (Qiagen), Trizol (Invitrogen), and viral RNA extraction was carried out according to the instructions;
[0027] 2. Amplification reaction system preparation
[0028]Take 15 μl of constant temperature amplification buffer, 10 μl of constant temperature amplification enzyme solution, and 25 μl of the RNA solution extracted in the above step 1, mix them into 50 μl of reaction solution, vortex and oscillate evenly, and inject into the constant temperature amplification reaction chip loaded with primers and probes;
[0029] The solvent of the constant temperature amplification buffer is sterile water, and the solute and concentration are as follows: 250mM Tris-HCL with pH 8.0, 80mM DTT, 15mM dNTP, 15mM rNTP, 60mM MgCl 2 , ...
Embodiment 3
[0033] Embodiment 3 comparative example setting
[0034] In the experiment, purchased commercial kits A and B (wherein, the kit is a RT-PCR detection kit, and kit B is a colloidal gold method detection kit) were used to verify the reliability of the detection results of the kit of the present invention. For the test kit of the present application is positive, while other comparative examples (kit A or B) are negative results, subsequent re-sampling of these sample cases is carried out for various test verifications. At the same time, the method of the present application is also compared to the clinically detected influenza B virus samples.
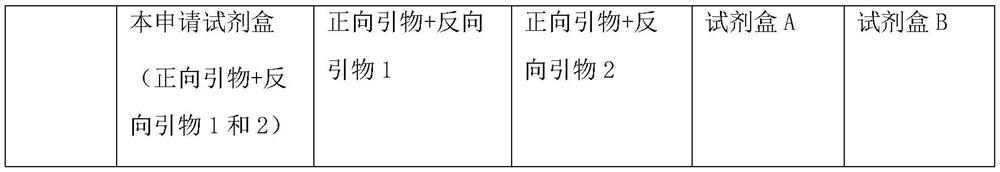
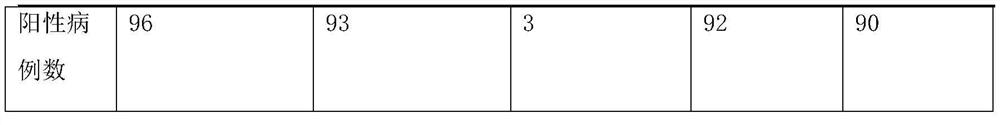
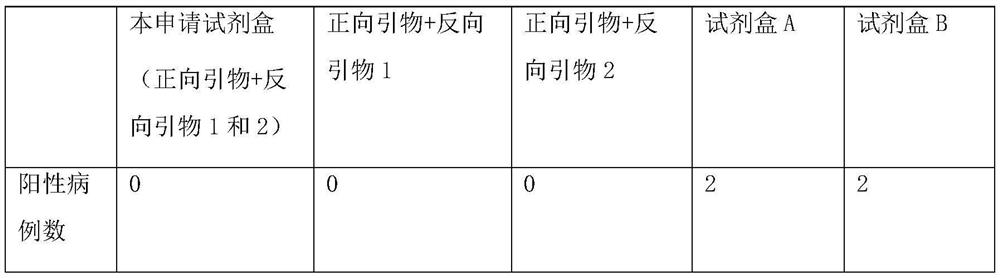
[0035] The clinical samples used in this application are all from hospitals in Heilongjiang Province, with a total of 783 cases. The test results are shown in Table 1 below:
[0036] Table 1: Comparison between the kits of this application and commercial kits
[0037]
[0038]
[0039] Through multiple verifications on the above-m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com