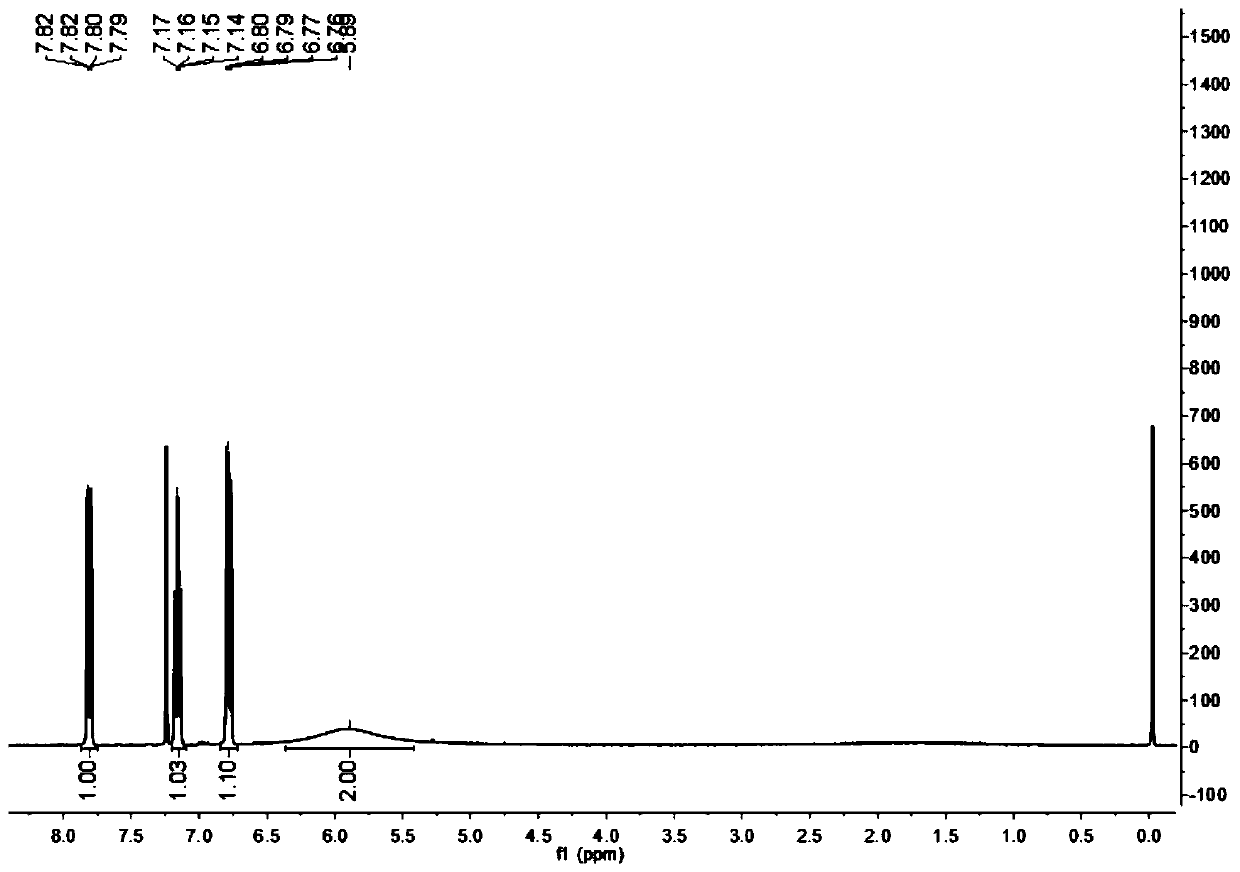
Method for synthesizing 4-fluoro-2-nitroaniline by micro-channel reactor
A technology of microchannel reactor and channel reactor, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of amino compounds, etc., can solve strong irritation and corrosion, yield is only 54%, increase production cost, etc. problem, achieve the effect of promoting the reaction yield, shortening the reaction cycle, and keeping a small amount
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A method for synthesizing 4-fluoro-2-nitroaniline in a microchannel reactor, the steps are:
[0032] a. Equipped with reactor and raw materials:
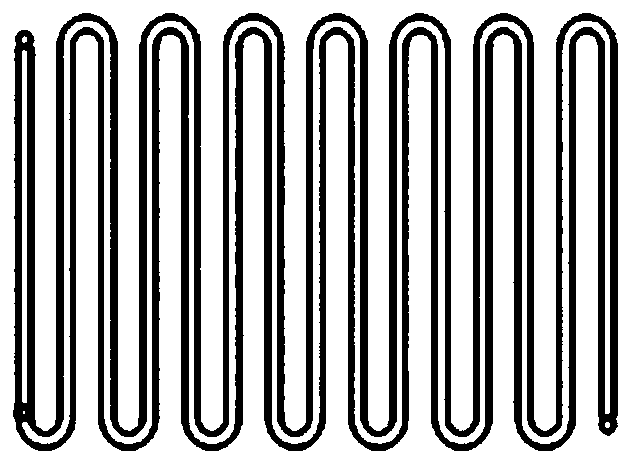
[0033] Equipped with Corning high-throughput continuous flow microchannel reactor (direct-flow microchannel preheating module + T-type microchannel enhanced mass transfer mixing module) as the reactor, the number of mixed reaction modules is determined according to the flow rate and reaction residence time, and the heat exchange medium For heat transfer oil;
[0034] The Corning high-throughput continuous flow microchannel reactor contains a direct-flow preheating module (that is, a direct-flow microchannel preheating module), a mass transfer-enhanced mixing module (that is, a T-shaped microchannel enhanced mass-transfer mixing module), an outlet ;
[0035] Be that the acetic acid-acetic anhydride solution of p-fluoroacetanilide of 20% by mass percentage concentration, sherwood oil and the nitric acid aqueous solution of 68...
Embodiment 2
[0041] A method for synthesizing 4-fluoro-2-nitroaniline in a microchannel reactor, the steps are:
[0042] a. Equipped with reactor and raw materials:
[0043] Equipped with Corning high-throughput continuous flow microchannel reactor (direct-flow microchannel preheating module + spherical microchannel enhanced mass transfer mixing module) as the reactor, the number of mixed reaction modules is determined according to the flow rate and reaction residence time, and the heat exchange medium For heat transfer oil;
[0044] The Corning high-throughput continuous flow microchannel reactor contains a direct-flow preheating module (ie, a direct-flow microchannel preheating module), a mass transfer-enhanced mixing module (ie, a spherical microchannel-enhanced mass-transfer mixing module), an outlet ;
[0045] Be that the acetic acid-acetic anhydride solution of p-fluoroacetanilide of 30% by mass percentage concentration, sherwood oil and the nitric acid aqueous solution of 68% by m...
Embodiment 3
[0051] A method for synthesizing 4-fluoro-2-nitroaniline in a microchannel reactor, the steps are:
[0052] a. Equipped with reactor and raw materials:
[0053] Equipped with Corning high-throughput continuous flow microchannel reactor (direct-flow microchannel preheating module + heart-shaped microchannel enhanced mass transfer mixing module) as the reactor, the number of mixed reaction modules is determined according to the flow rate and reaction residence time, and the heat exchange medium For heat transfer oil;
[0054] The Corning high-throughput continuous flow microchannel reactor contains a direct-flow preheating module (ie, a direct-flow microchannel preheating module), a mass transfer-enhanced mixing module (ie, a heart-shaped microchannel-enhanced mass-transfer mixing module), an outlet ;
[0055] Be that the acetic acid-acetic anhydride solution of p-fluoroacetanilide of 35% by mass percentage concentration, sherwood oil and the nitric acid aqueous solution of 68...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com