Preparation method of m-phenylenediamine
A technology of m-phenylenediamine and nitroaniline, which is applied in the preparation of carboxylic acid amides, organic compounds, nitro compounds, etc. It can solve the problems of small-scale experiments, difficult temperature control, and the explosive nature of dinitrobenzene and other problems to achieve the effect of avoiding the explosive compound dinitrobenzene, stabilizing the production process, and avoiding dangerous processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
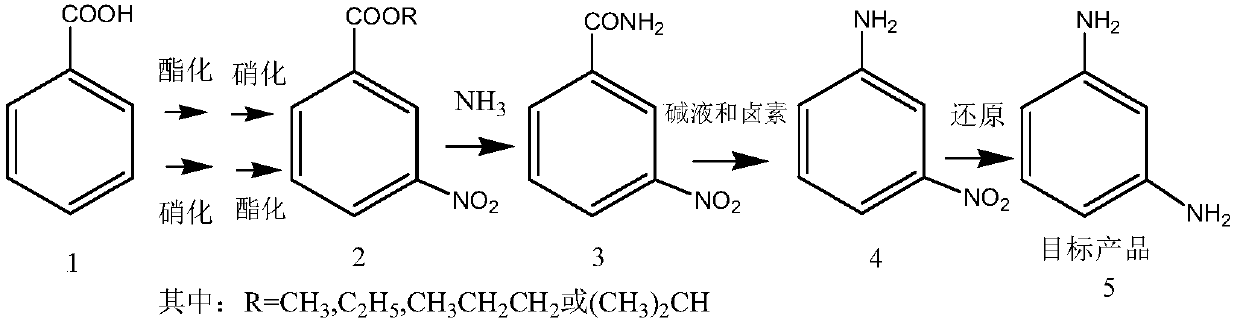
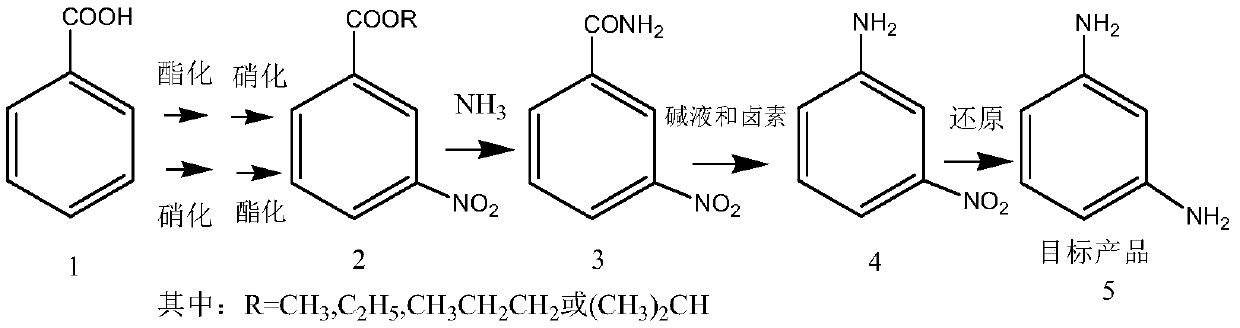
[0034] A kind of preparation method of m-phenylenediamine, this method takes benzoic acid as starting raw material, first reacts esterification with alcohol reagent under the action of acid, then nitrates to obtain m-nitrobenzoate, or with benzoic acid As the starting material, it is first nitrated, and then esterified with alcohol reagents under the action of acid to obtain m-nitrobenzoate; and then ammonolysis of m-nitrobenzoate to obtain 3-nitrobenzoate Amide, m-nitroaniline is prepared after reacting with hypohalite or halogen in an alkaline solvent, and the m-phenylenediamine is obtained after reduction.
[0035] The reaction process is as follows:
[0036]
[0037] Wherein, compound 1 is benzoic acid, compound 2 is m-nitrobenzoate (or 3-nitrobenzoate), compound 3 is 3-nitrobenzamide (or m-nitrobenzamide), Compound 4 is m-nitroaniline (or 3-nitroaniline), and target product 5 is m-phenylenediamine.
[0038] As the preferred technical scheme of the present invention, ...
Embodiment 1
[0062] 61g of benzoic acid (compound 1) was dissolved in 200g of methanol, 2g of sulfuric acid was added, the temperature was raised to reflux, and the generated water was continuously evaporated. After the temperature reached 66.5°C, the methanol was evaporated to dryness, and 180ml of dichloroethane was added, and the temperature was raised to 30°C. Add 35g of fuming nitric acid dropwise, detect with thin-layer chromatography, after methyl benzoate disappears, add 100g of ice water, neutralize with alkali, evaporate the solvent, filter and wash, and obtain 81.1g of methyl m-nitrobenzoate after recrystallization (compound 2). Yield 89.6%.
Embodiment 2
[0064] Dissolve 61g of benzoic acid (compound 1) in 200g of ethanol, add 2g of p-toluenesulfonic acid, heat up and reflux, and continuously evaporate the formed water. After the temperature reaches 79-80°C, evaporate the ethanol to dryness, and add 180ml of dichloroethane , the temperature was raised to 30°C, 35g of fuming nitric acid was added dropwise, detected by thin-layer chromatography, after the methyl benzoate disappeared, 100g of ice water was added, the lower organic liquid layer was separated, washed, the solvent was distilled off, filtered and washed, and weighed After crystallization, 82.9 g of methyl m-nitrobenzoate (compound 2) were obtained. Yield 91.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com