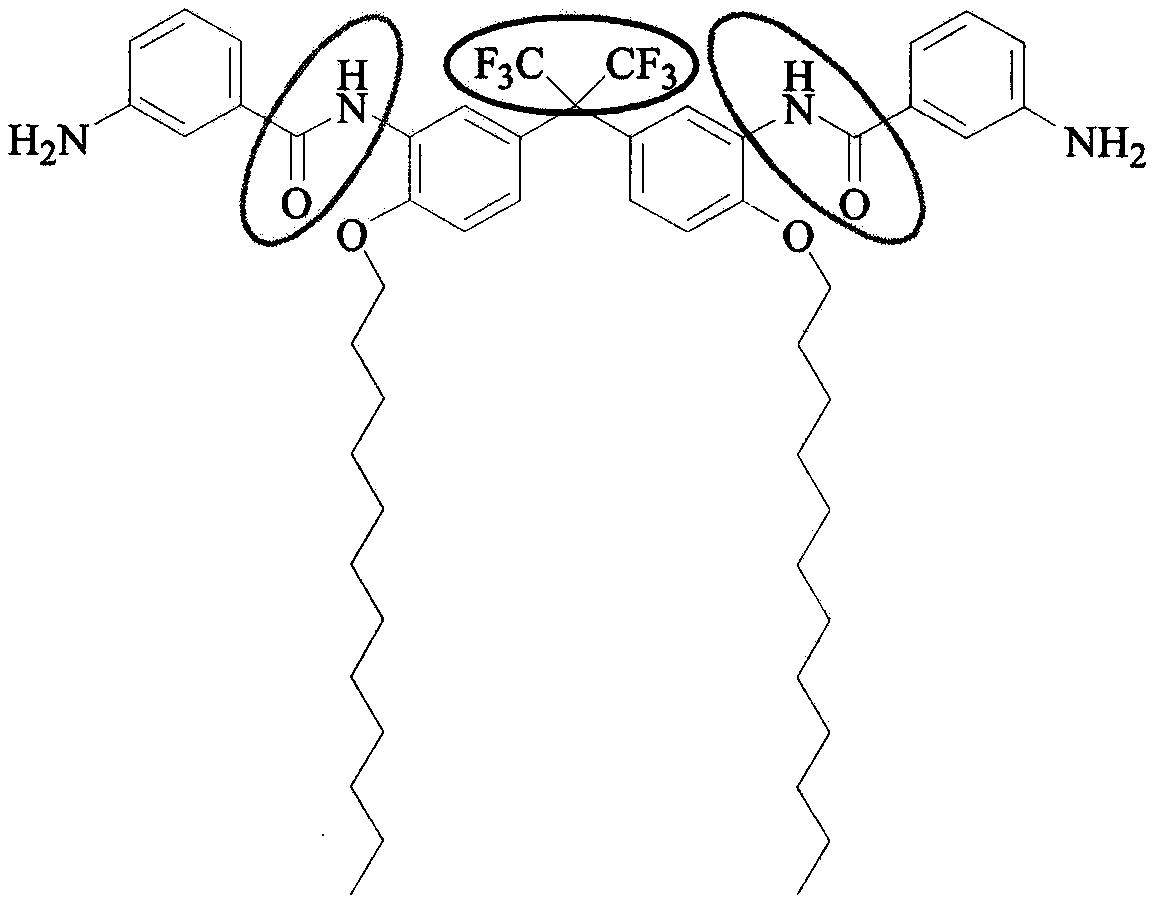
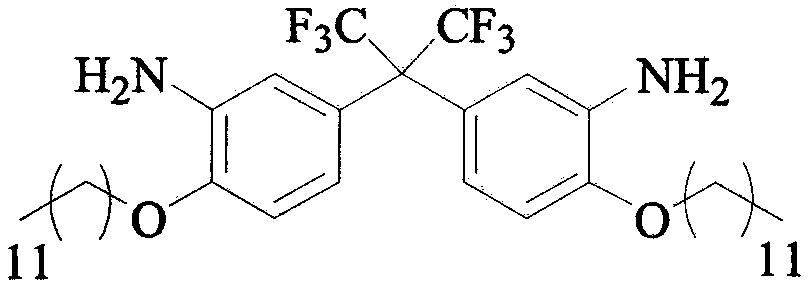
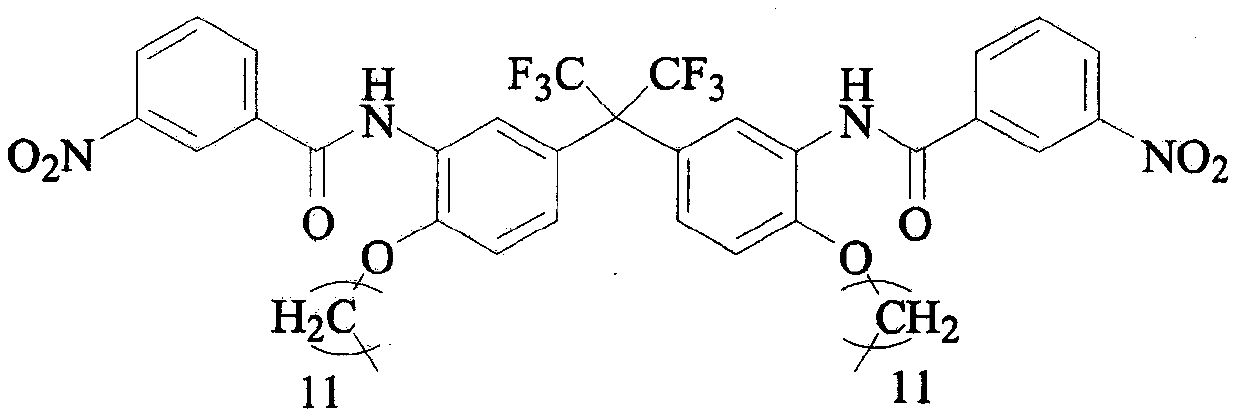
Preparation method of C12 side-chain substituted fluorine-containing diamine monomer
A fluorine-containing diamine, C12-FN technology, applied in the direction of carboxylic acid amide preparation, organic compound preparation, chemical instruments and methods, etc., can solve the problems of high molecular weight of diamine monomers, restrictions on the development of polyimide, nitro Reducing problems such as difficulty, achieving high synthesis yield, improving light transmittance, reducing order and symmetry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Step 1: Add 1.2L of DMAC, 1000g of 2,2-bis(3-amino-4-phenol sodium)hexafluoropropane and 1210g of bromo C12 alkane to a 10L four-necked reaction flask, turn on stirring and heating, and maintain React at 80°C for 2-3h. After the raw material has reacted, turn off the heating, pour out the reaction solution, and filter while it is hot. The upper filter cake is the by-product sodium bromide, which is collected and stored. The filtrate is naturally cooled to room temperature, and then added to 2.4 After quenching in L water, there will be a large amount of solids appearing at this time, suction filter, and then wash the filter cake with 3.5L of water. After pumping dry, the filter cake is vacuum dried to obtain 1542g of pure C12-FN.
[0028] Step 2: Add 1542g C12-FN and 550mL NMP to a 10L four-necked reaction flask, turn on the stirring, and then slowly drop a mixture of 812g m-nitrobenzoyl chloride and 550mL NMP into it, and control the reaction liquid during the dropping. T...
Embodiment 2
[0031] Step 1: Add 24.3L of DMAC, 1000g of 2,2-bis(3-amino-4-sodium phenolate) hexafluoropropane and 6051g of bromo-C12 alkane to a 30L double-layer glass reactor in sequence, turn on stirring and heating, Keep at 166°C for 2-3h. After the reaction of the raw materials, turn off the heating, release the reaction liquid, and filter while it is hot. The upper filter cake is the by-product sodium bromide, which is collected and stored. The filtrate is naturally cooled to room temperature, and then added to 48.6 After quenching in L water, there will be a large amount of solids appearing at this time, suction filter, and then wash the filter cake with 3.5L of water. After pumping dry, the filter cake is vacuum dried to obtain 1627g of pure C12-FN.
[0032] Step 2: Add 1627g of C12-FN and 11.5L of NMP to the 30L double-layer glass reactor, turn on the stirring and jacket to cool down, when the temperature of the reaction solution drops to 0℃, slowly add 4286g of m-nitrobenzene dropwise...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com