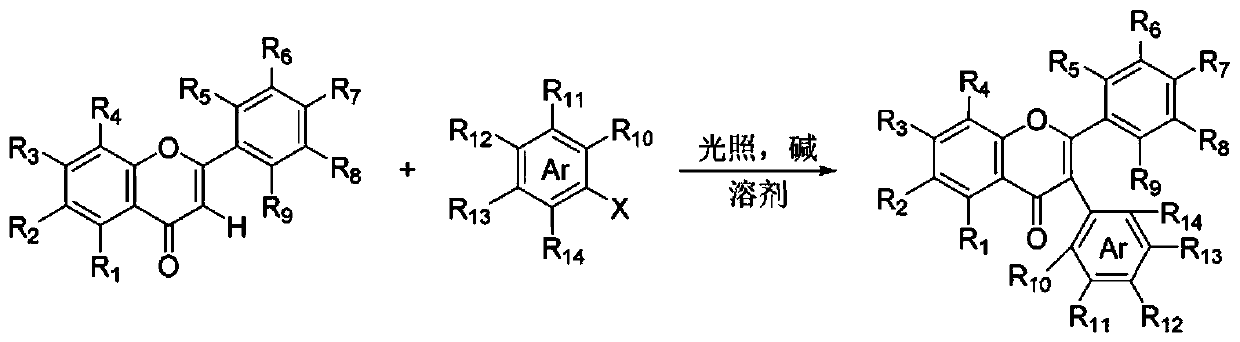
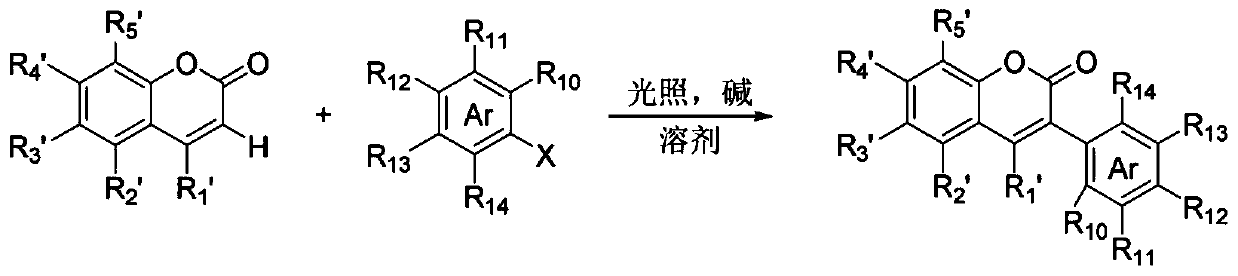
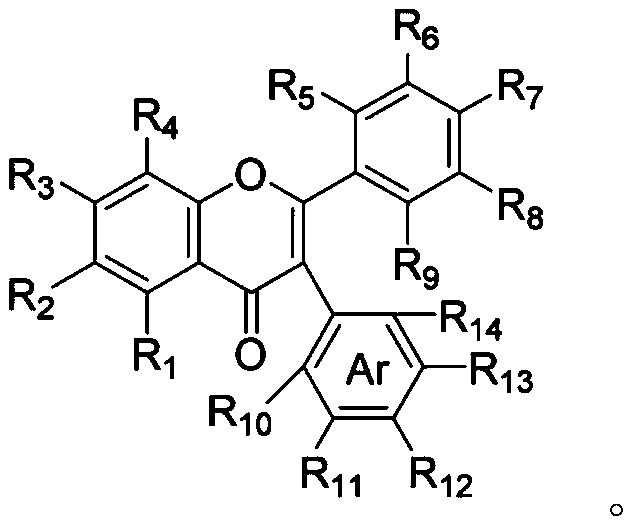
Method for synthesizing 3-aryl flavone or coumarin compound through photo-initiation, and applications of 3-aryl flavone or coumarin compound
A technology of aryl coumarin and aryl flavonoids, which is applied in the direction of chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problems of high difficulty and low synthesis efficiency, achieve few by-products, simple method, The effect of high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Synthesis.
[0041] Under a nitrogen atmosphere, 3',4'-dihydroxyflavone (0.1 mmol), iodobenzene (0.1 mmol), cesium carbonate (0.2 mmol) were dissolved in dimethyl sulfoxide (1 mL), After the addition, the reactant was placed at 25°C, irradiated with 450nm light for 26 hours, quenched with dilute hydrochloric acid (1N), the aqueous phase was extracted three times with ethyl acetate (15 ml × 3), and the organic phases were combined , washed with water and saturated brine successively, dried over anhydrous sodium sulfate, concentrated and then column chromatographed to obtain 3-arylflavone derivatives (26.4 mg, 80% yield).
Embodiment 2
[0043] Synthesis.
[0044] Under a nitrogen atmosphere, dissolve 5,4'-dihydroxyflavone (0.1 mmol), p-bromoacetophenone (0.3 mmol), and potassium carbonate (0.5 mmol) in N,N-dimethylformamide (1 ml), after the addition, the reactant was placed under the condition of 50° C., irradiated at 390 nm for 16 hours, quenched the reaction with dilute hydrochloric acid (1N), and the aqueous phase was extracted three times with ethyl acetate (15 ml×3 ), the organic phases were combined, washed with water and saturated brine successively, dried over anhydrous sodium sulfate, concentrated and then column chromatographed to obtain 3-arylflavone derivatives (31.6 mg, 85% yield).
Embodiment 3
[0046] Synthesis.
[0047] Under nitrogen atmosphere, 4'-hydroxy-6-methylflavone (0.1 mmol), m-chlorobenzonitrile (0.5 mmol), potassium bicarbonate (0.4 mmol) were dissolved in N,N-dimethyl In acetamide (1 ml), after the addition was complete, the reactant was placed at 0°C, irradiated at 410 nm for 16 hours, quenched with dilute hydrochloric acid (1N), and the aqueous phase was extracted three times with ethyl acetate (15 ml ×3), the organic phases were combined, washed successively with water and saturated brine, dried over anhydrous sodium sulfate, concentrated and then column chromatographed to obtain 3-arylflavone derivatives (27.5 mg, 78% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com