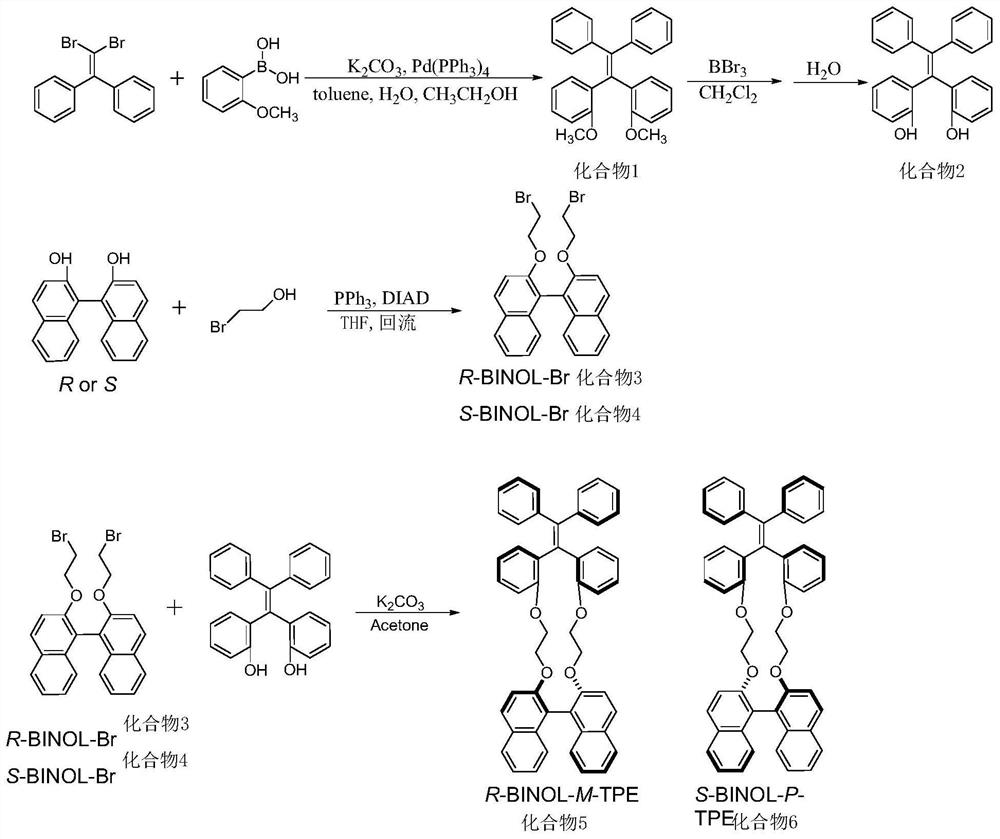
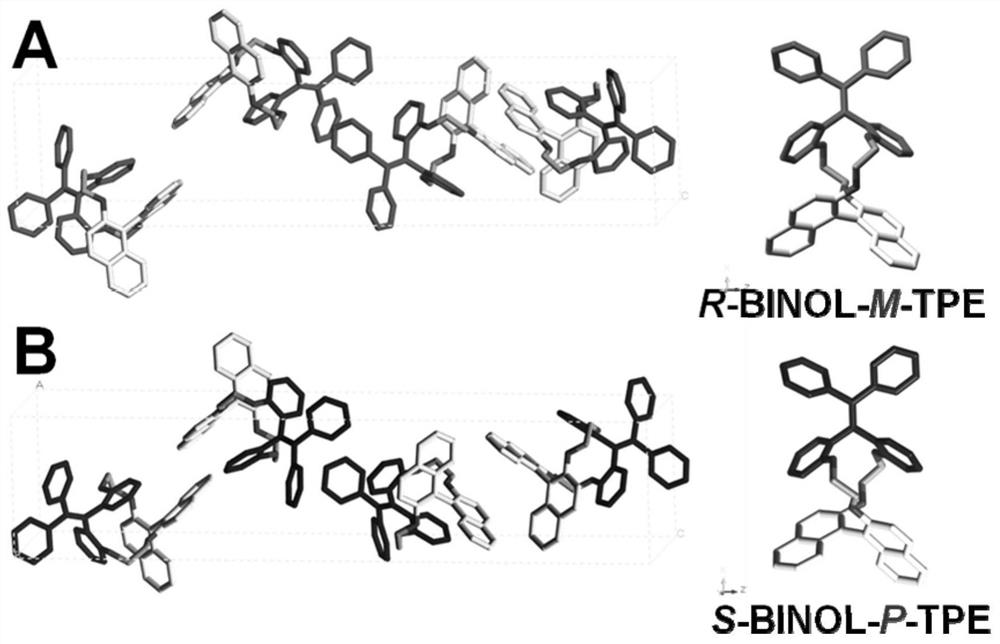
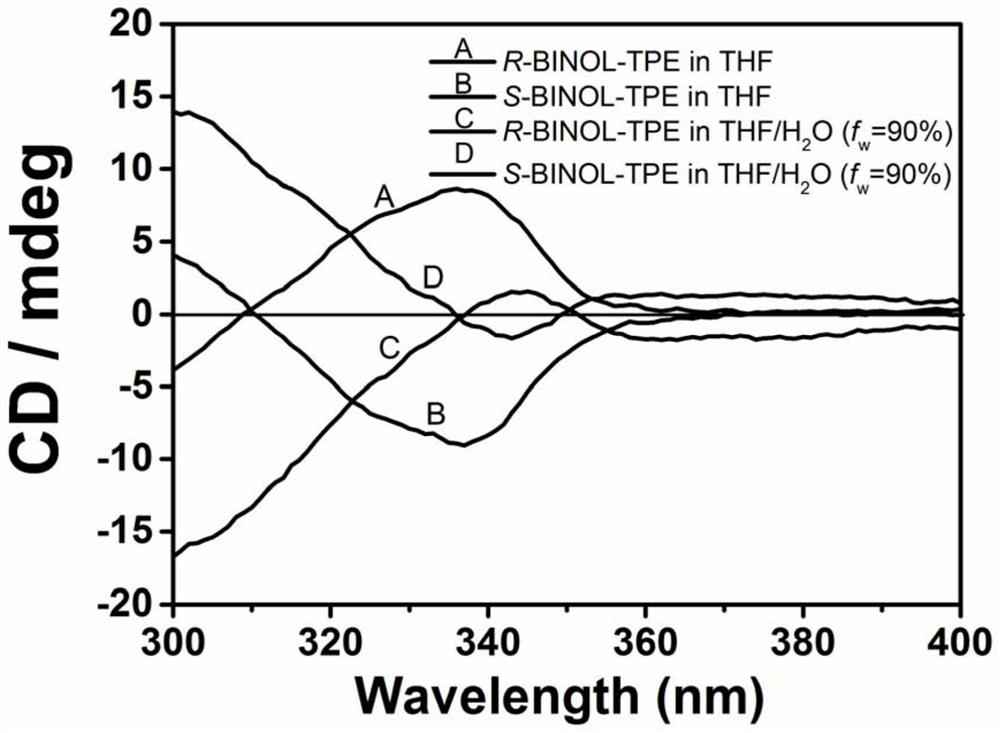
A kind of chiral tetraphenylethylene and its synthetic method
A tetraphenylethylene and synthetic method technology, applied in chemical instruments and methods, organic chemistry, luminescent materials, etc., can solve problems such as difficult to obtain CPL materials, achieve excellent aggregation-induced luminescence performance, convenient synthesis, and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The preparation method of the α,α'-dimethoxytetraphenylethylene is obtained by performing Suzuki reaction on dibromostilbene and α-methoxyphenylboronic acid. The process of the Suzuki reaction is: under the protection of an inert gas, dissolve dibromostilbene, α-methoxyphenylboronic acid, tetrakis(triphenylphosphine)palladium, and potassium carbonate in a mixed solvent and raise the temperature to 90-95°C react. Suzuki reaction time is 16 ~ 20h. The mixed solvent is a mixture of toluene, water and ethanol, wherein, toluene is used as an organic phase solvent, water is used as an inorganic phase solvent, and ethanol is used as a phase transfer catalyst. The volume ratio of water to ethanol is 1:1, and the volume ratio of toluene, water and ethanol It is 10:1:1. The molar ratio of dibromostilbene, α-methoxyphenylboronic acid, tetrakis(triphenylphosphine)palladium, and potassium carbonate is 1:5:0.1:5. The ratio of dibromostilbene to toluene is 1 mmol: 15 mL.
[0045]T...
Embodiment 1
[0050] Embodiment 1α, the synthesis of α '-dimethoxytetraphenylethylene
[0051] Dibromostilbene (2.028g, 6mmol), α-methoxyphenylboronic acid (4.56g, 30mmol), potassium carbonate (4.416g, 30mmol), palladium tetrakistriphenylphosphine (693mg, 0.6mmol) In a 250mL round bottom bottle; under nitrogen protection, add 100mL toluene, 2mL water, 2mL ethanol, stir until dissolved, raise the reaction temperature to 90°C, and continue the reaction for 18h; after the reaction, extract with dichloromethane (3×50mL) , combine the organic phases; use anhydrous magnesium sulfate to dry and remove the solvent; use n-hexane / dichloromethane (90 / 10) as the mobile phase for column chromatography to obtain a white solid, which is α,α′-dimethyl Oxytetraphenylethylene (referred to as compound 1), yield 73%.
Embodiment 2
[0052] Example 2α, the synthesis of α'-dihydroxytetraphenylethylene
[0053] Compound 1 (0.392g, 1.0mmol) was placed in a 50mL reactor; under nitrogen protection, 10mL of dry dichloromethane was added, stirred until dissolved; cooled to -65°C, stirred for 15min; added 4.0mL of boron tribromide Dichloromethane solution (1.0mol / L), take out the reactor, put it at room temperature; stir at room temperature for 12h; take 100mL deionized water and place it in a 250mL round bottom bottle; bubble nitrogen for 10min; pour the liquid in the reactor put into a round-bottom flask under nitrogen protection, and stir for 1 h; extract with dichloromethane (3×50 mL), combine the organic phases; dry over anhydrous magnesium sulfate and remove the solvent to obtain a white solid, which is α,α′-di Hydroxytetraphenylethylene (referred to as compound 2), yield 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com