A near-infrared fluorescent probe for detecting cys in the brain of depressed mice and its synthesis method and application
A technology of fluorescent probes and synthesis methods, applied in the field of synthesis of near-infrared fluorescent probes, to achieve the effect of simple operation methods, simple operation, and universal applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] A method for preparing a novel near-infrared fluorescent probe for detecting Cys in cells and living bodies, comprising the following steps:
[0038] Probe structure:
[0039]
[0040] Probe synthesis method:
[0041]
[0042] The structural formula of Changsha Red of the present application is as follows:
[0043]
Embodiment 1
[0047] Synthesis of compound b: under nitrogen protection conditions, 20mL DMF and 20mlCH 2 Cl 2 After mixing evenly, add 18ml POCl dropwise at 0°C 3 and 17mlCH 2 Cl 2 After the dropwise addition was completed, 2.65ml of cyclohexanone was added dropwise. After the dropwise addition, the reaction was transferred to an oil bath and reacted at 40°C for 3h. The reaction was quickly poured into crushed ice and kept overnight at 0°C. A yellow solid was obtained by suction filtration.
[0048] Synthesis of compound e: under nitrogen protection, 600 μL iodoethane and 800 μL indole were dissolved in 3 ml toluene, reacted at 80° C. for 12 h, cooled, precipitated and filtered to obtain a purple solid.
[0049] Synthesis of compound f: Mix 1.63g of product e, 0.45g of product b, 0.6g of sodium acetate, and 10ml of acetic anhydride under nitrogen protection, stir at room temperature for 24 hours in the dark, pour it into ether, and leave it to precipitate with a metallic luster green ...
Embodiment 2
[0054] The optical properties of the probe prepared in Example 1 were tested.
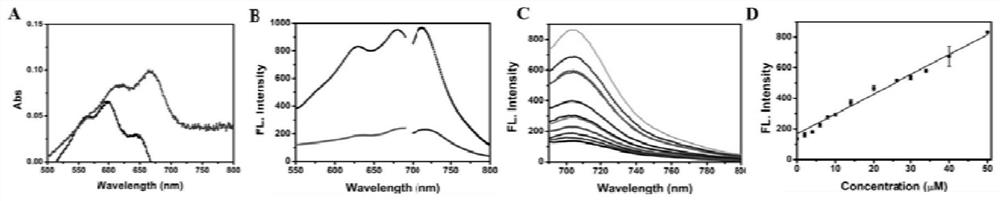
[0055] After adding Cys, the probe showed an absorption peak at about 700 nm ( figure 1 Middle A). Such as figure 1 As shown in B and C, under single-photon 590nm excitation, the fluorescence intensity of the probe at 710nm is weak, but after the probe is incubated with Cys, the fluorescence intensity of the probe at 710nm is significantly enhanced, and the fluorescence intensity varies with Cys concentration increased and enhanced. Such as figure 1 As shown in D, the linear range of the probe is 0-50 μM, and the detection limit is 0.45 μM.
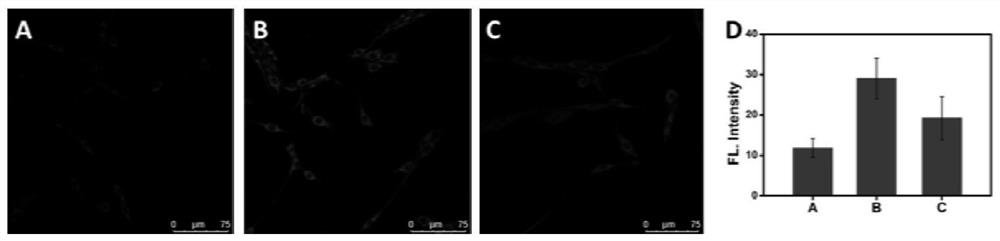
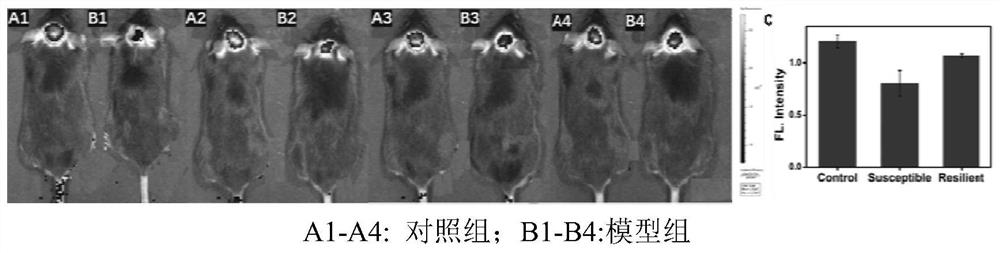
[0056] Experiments of the invention in cells. The present invention first incubates PC12 cells with DTT (dithiothreitol) (increasing intracellular Cys) for 15 minutes, then adds a probe (10 μM) and then incubates for 2 minutes, and then uses PBS (phosphate-buffered saline) to remove residual extracellular The probes were washed away for fluorescence imag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com