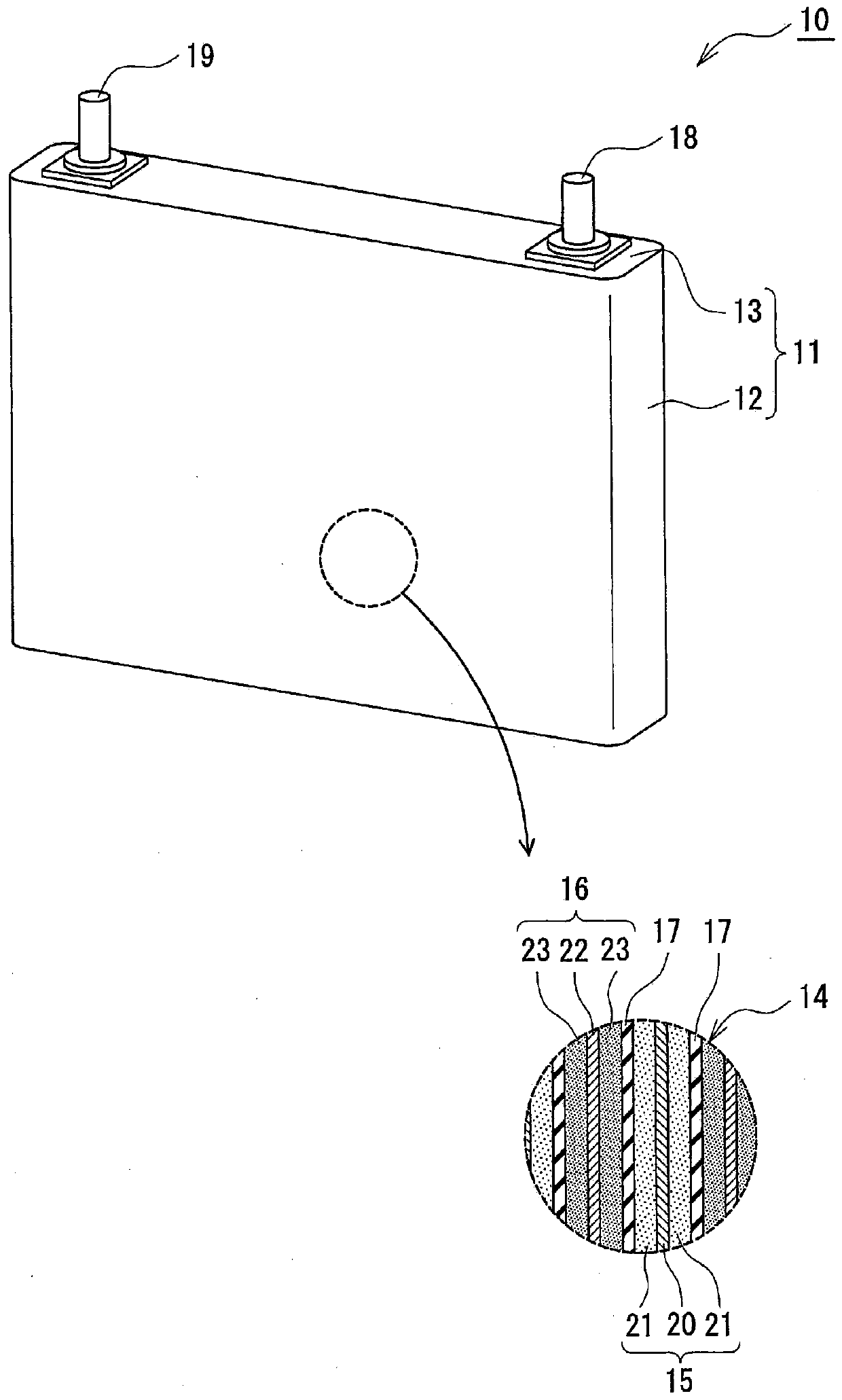
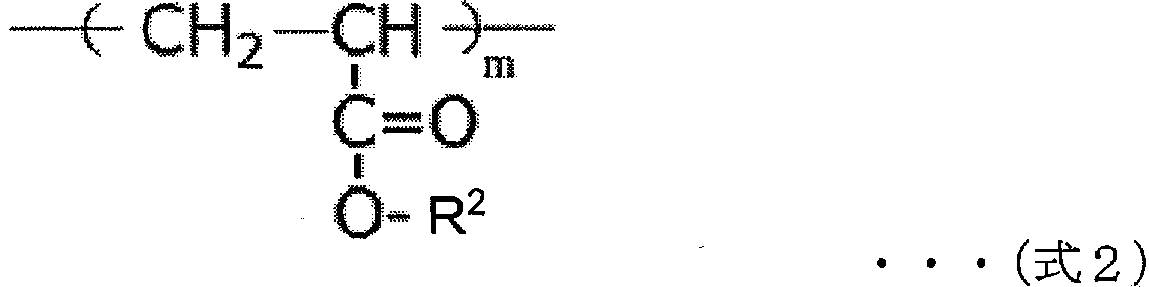
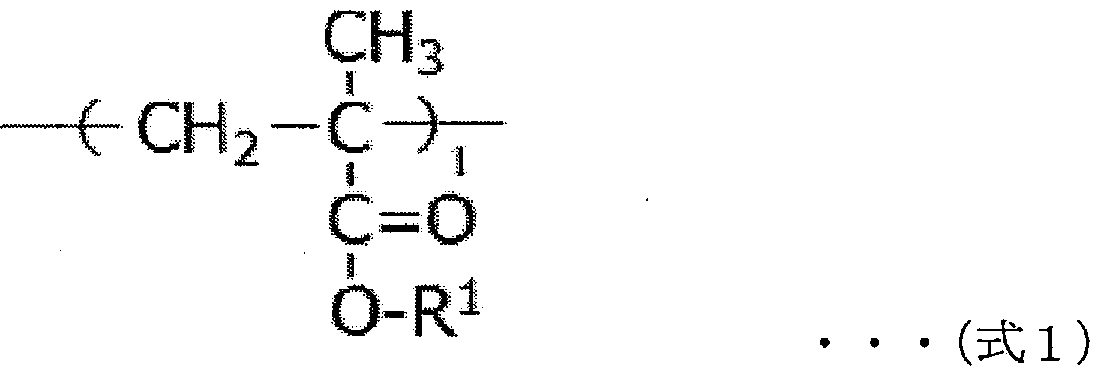Negative electrode for non-aqueous electrolyte secondary battery, and non-aqueous electrolyte secondary battery
A non-aqueous electrolyte and secondary battery technology, which is applied to non-aqueous electrolyte battery electrodes, non-aqueous electrolyte batteries, non-aqueous electrolytes, etc. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0094] The present disclosure will be further described below through examples, but the present disclosure is not limited to these examples.
Synthetic example 1
[0096]170 g of ion-exchanged water was poured into a reaction container equipped with a stirrer, a reflux tube, a dropping funnel, a thermometer, and a nitrogen gas introduction tube, and the temperature was raised to 70° C. while stirring under a nitrogen gas flow. Then, an aqueous solution obtained by dissolving 0.5 g of ammonium persulfate in 5 g of ion-exchanged water was added. 37 g of methyl methacrylate, 60 g of n-butyl acrylate, and 3 g of acrylic acid were put into the dropping funnel, and were dropped into the reaction vessel at a constant rate over 3 hours. After completion of the dropwise addition, aging was performed at 70° C. for 3 hours. Next, after cooling to 40° C., 14.6 ml of a 1N aqueous sodium hydroxide solution was added to partially neutralize the carboxylic acid groups derived from acrylic acid. After distilling off water from the reaction vessel under reduced pressure, a small amount of aggregate was removed by filtration through a mesh, and the solid ...
Synthetic example 2~21
[0098] Using the monomer components and neutralizing agents shown in Table 1, an emulsion binder was obtained by the soap-free emulsion polymerization method in the same manner as in Synthesis Example 1 at the monomer compounding ratio shown in Table 1. In addition, the solid content concentration of the emulsion binder was adjusted to 50%. In Synthesis Examples 19 and 20, 2 parts by weight of a polyoxyethyl sodium lauryl sulfate aqueous solution having a solid content concentration of 25% was added to the ion-exchanged water thrown into the reaction container as a surfactant. In addition, EHA of the synthesis example 20 represents 2-ethylhexyl acrylate.
[0099] [Measurement of solid content]
[0100] The measurement was performed in an automatic mode (60 seconds) at a temperature of 120° C. using an infrared moisture meter (kett FD-240, manufactured by Kett Science Institute), and the solid content of the emulsion binder was calculated.
[0101] [Calculation of Tg]
[010...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com