Gallium arsenide etching method
A gallium arsenide, chemical reaction technology, applied in the field of solar cells, can solve the problems of difficult to control the etching rate, incomplete etching, waste of raw materials, etc., and achieve the effect of easy control of the etching rate and high precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
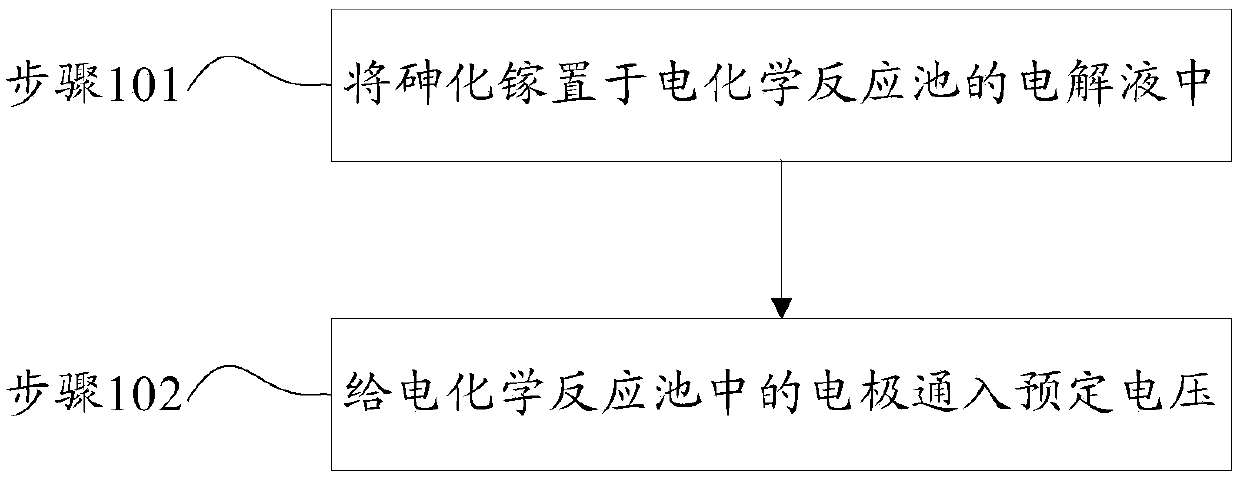
[0049] Step 1: put gallium arsenide into the electrolyte solution of the electrochemical reaction cell, and the electrolyte solution includes hydrochloric acid, copper bromide and water. Wherein, the pH value of the electrolytic solution is 0.7-1, and the concentration of copper bromide is 140 g / L.
[0050] Step 2: Calculate the etching time according to the target etching amount of gallium arsenide, calculate the etching rate according to the etching time, and obtain the etching voltage value.
[0051] The electrodes are energized, the control voltage is the above etching voltage value, and the bromine gas released from the electrochemical reaction cell is collected. Gallium arsenide is etched by an electrochemical reaction.
[0052] During the electrochemical reaction, the pH value of the electrolyte and the concentration of copper bromide were detected. According to the test results, determine whether to add acid, metal bromide and water to the electrochemical reaction ce...
experiment example 2
[0056] Step 1: put gallium arsenide into the electrolyte solution of the electrochemical reaction cell, the electrolyte solution is composed of hydrochloric acid, copper bromide and water, and the pH value of the electrolyte solution is 0.7-1, and the concentration of copper bromide is 150g / L.
[0057] Step 2: Calculate the etching time according to the target etching amount of gallium arsenide, calculate the etching rate according to the etching time, and obtain the etching voltage value.
[0058] The electrodes are energized, the control voltage is the above etching voltage value, and the bromine gas released from the electrochemical reaction cell is collected. Gallium arsenide is etched by an electrochemical reaction.
[0059] During the electrochemical reaction, the pH value of the electrolyte and the concentration of copper bromide were detected. According to the test results, determine whether to add acid, metal bromide and water to the electrochemical reaction cell to ...
experiment example 3
[0063] Step 1: put gallium arsenide into the electrolyte solution of the electrochemical reaction cell, the electrolyte solution is composed of hydrochloric acid, copper bromide and water, and the pH value of the electrolyte solution is 0.7-1, and the concentration of copper bromide is 160g / L.
[0064] Step 2: Calculate the etching time according to the target etching amount of gallium arsenide, calculate the etching rate according to the etching time, and obtain the etching voltage value.
[0065] The electrodes are energized, the control voltage is the above etching voltage value, and the bromine gas released from the electrochemical reaction cell is collected. Gallium arsenide is etched by an electrochemical reaction.
[0066] During the electrochemical reaction, the pH value of the electrolyte and the concentration of copper bromide were detected. According to the test results, determine whether to add acid, metal bromide and water to the electrochemical reaction cell, so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com