Ba-Mn perovskite-type cobalt-based catalyst for hydrogen production by autothermal reforming of acetic acid
A cobalt-based catalyst and perovskite-type technology, applied in the field of hydrogen production from autothermal reforming of acetic acid, can solve problems such as catalyst deactivation, achieve the effects of reducing activation energy, improving stability, and suppressing intermediate products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0025] Weigh 1.416g of Co(NO 3 ) 2 ·6H 2 O, 3.824g of Ba(CH 3 COO) 2 and 5.358g of 50wt% Mn(NO 3 ) 2 solution, add 30ml of deionized water to prepare solution #1; then weigh 10.971g of citric acid C 6 h 8 o 7 ·H 2 O, add 30ml of deionized water to prepare solution #2; slowly add solution #1 dropwise to solution #2, and stir at 60°C, after 2.5 hours, the solution gradually turns into a colloid, and put it in an oven In the process, dry at 105°C for 12 hours; heat the dried sample to 1000°C at a rate of 10°C / min in a tube furnace, and bake it for 4 hours to obtain the catalyst CDUT-CMC-1 of the present invention. The molar composition of oxides is (BaO) 0.43 (MnO 1.5 ) 0.43 (CoO 1.5 ) 0.14 , and the weight percentage in terms of oxides is composed as follows: the content of barium oxide is 60.4%, the content of manganese oxide is 29.5%, and the content of cobalt oxide is 10.0%.
[0026] The reactivity evaluation of autothermal reforming of acetic acid was carried ...
Embodiment 1
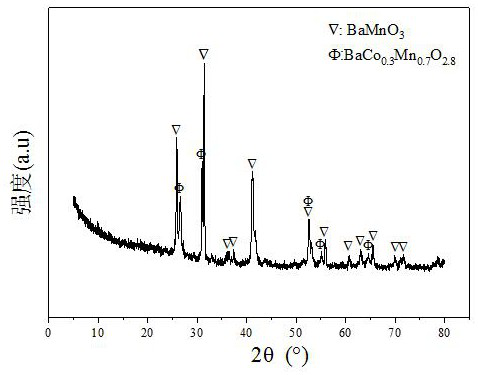
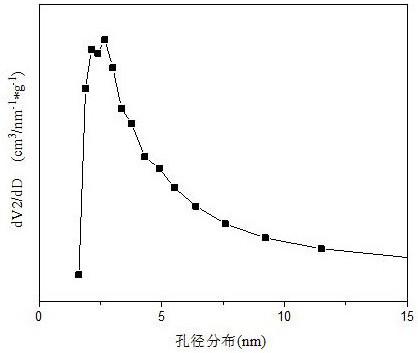
[0029] Weigh 2.811g of Co(NO 3 ) 2 ·6H 2 O, 3.854g of Ba(CH 3 COO) 2 and 21.604g of 50wt% Mn(NO 3 ) 2 solution, add 30ml of deionized water to prepare solution #1; then weigh 26.828g of citric acid C 6 h 8 o 7 ·H 2 O, add 30ml of deionized water to prepare solution #2; slowly add solution #1 dropwise to solution #2, and stir at 60°C, after 2.5 hours, the solution gradually turns into a colloid, and put it in an oven , dried at 105°C for 12 hours. The dried sample was heated to 1000°C at a rate of 10°C / min in a tube furnace, and calcined for 4 hours to obtain the catalyst CDUT-CMC-2 of the present invention. Its typical XRD spectrum is shown in the attached figure 1 shown, with BaMnO containing 3 and BaMn 0.7 co 0.3 o 2.8 structure of Ba-Mn perovskite-type Ba-Mn-Co-O composite oxide cobalt-based catalysts, and at the same time, formed such as attached figure 2 The pore size distribution of the shown mesopores; the molar composition of the catalyst in terms of ox...
Embodiment 2
[0032] Weigh 2.821g of Co(NO 3 ) 2.6 ·H 2 O, 5.759g of Ba(CH 3 COO) 2 and 16.138g of 50wt% Mn(NO 3 ) 2 solution, add 30ml of deionized water to prepare solution #1; then weigh 24.375g of citric acid C 6 h 8 o 7 ·H 2 O, add 30ml of deionized water to prepare solution #2; slowly add solution #1 dropwise to solution #2, and stir at 60°C, after 2.5 hours, the solution gradually turns into a colloid, and put it in an oven In the process, dry at 105°C for 12 hours; heat the dried sample to 1000°C at a rate of 10°C / min in a tube furnace, and bake it for 4 hours to obtain the catalyst CDUT-CMC-3 of the present invention, typically structure as attached figure 1 shown, with BaMnO containing 3 and BaMn 0.7 co 0.3 o 2.8 Structured Ba-Mn perovskite-type Ba-Mn-Co-O composite oxide cobalt-based catalyst, the molar composition in terms of oxide is: (BaO) 0.29 (MnO 1.5 ) 0.58 (CoO 1.5 ) 0.13 , and the weight percentage in terms of oxides is composed as follows: the content ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com