Barium aluminate spinel composite oxide cobalt-based catalyst for hydrogen production by autothermal reforming of acetic acid
A cobalt-based catalyst, aluminum spinel technology, applied in metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, chemical/physical process and other directions, can solve problems such as catalyst deactivation, achieve enhanced Effects of thermal stability, prevention of migration and sintering, improvement of conversion rate and hydrogen production rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0029] Weigh 1.428g of Co(NO 3 ) 2 ·6H 2 O, 5.252g of Ba(NO 3 ) 2 and 3.769g of Al(NO 3 ) 3 ·6H 2 O, dissolved in 20.0mL deionized water solution, heated to 60°C in water bath and stirred to prepare solution #1; dissolved 7.366g citric acid and 2.176g ethylene glycol in 20.0mL deionized water solution, heated to 60°C in water bath and stir to prepare solution #2; mix and stir solution #1 and solution #2 in a water bath at 60°C to form a gel and dry at 105°C for 12 hours, and the dried catalyst is calcined at 700°C for 4 hours, namely The catalyst CA-4B of the present invention is obtained. The weight percentage of the catalyst in terms of oxides is as follows: the content of barium oxide is 77.0%, the content of aluminum oxide is 12.8%, and the content of cobalt oxide is 10.2%.
[0030] The reactivity evaluation of autothermal reforming of acetic acid was carried out in a continuous flow fixed bed reactor. Grind and tablet the catalyst, then sieve it into 20-40 mesh p...
Embodiment 1
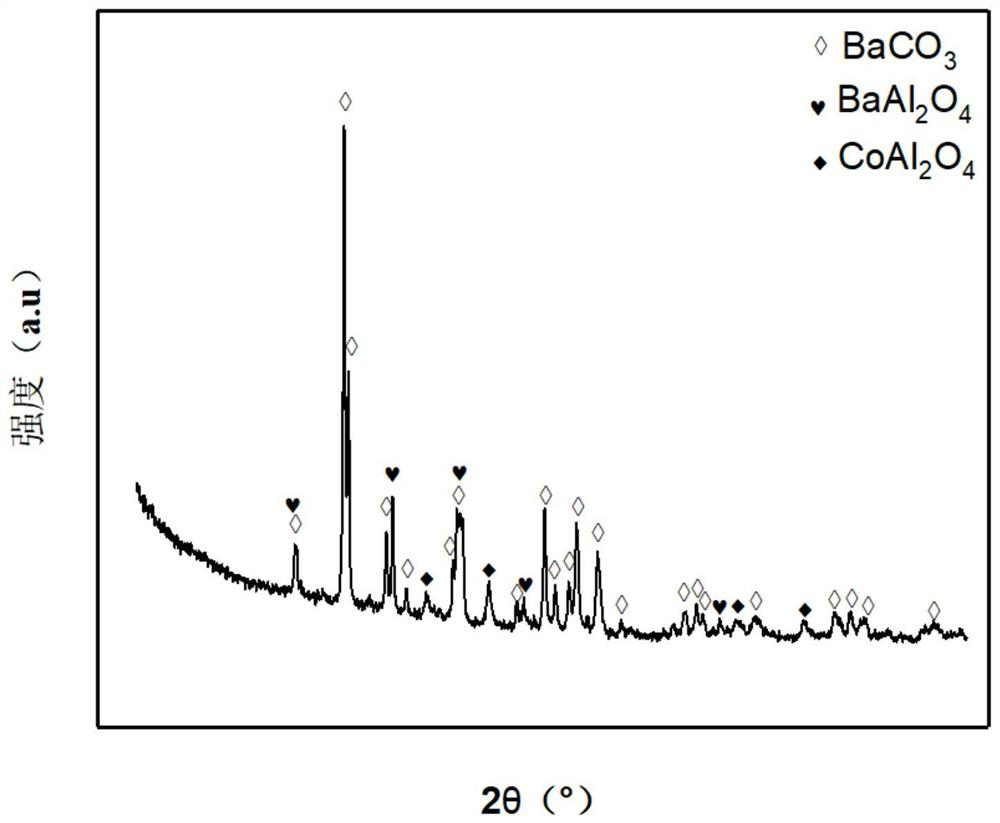
[0033] Weigh 1.459g of Co(NO 3 ) 2 ·6H 2 O, 4.586g of Ba(NO 3 ) 2 and 6.582g of Al(NO 3 ) 3 ·6H 2 O, dissolved in 20.0mL deionized water solution, heated to 60°C in water bath and stirred to prepare solution #1; dissolved 8.428g citric acid and 2.489g ethylene glycol in 20.0mL deionized water solution, heated to 60°C in water bath and stir to prepare solution #2; mix and stir solution #1 and solution #2 in a water bath at 60°C to form a gel and dry at 105°C for 12 hours, and the dried catalyst is calcined at 700°C for 4 hours, namely Get the Co-Ba-Al-O active center, BaCO 3 is the skeleton and has BaAl 2 o 4 、CoAl 2 o 4 Cobalt barium aluminum catalyst CA-2B with spinel phase, its typical structure is as attached figure 1 shown. The weight percentage of the catalyst in terms of oxides is as follows: the content of barium oxide is 67.2%, the content of aluminum oxide is 22.4%, and the content of cobalt oxide is 10.4%.
[0034] The CA-2B catalyst was used in the aut...
Embodiment 2
[0036] Weigh 1.509g of Co(NO 3 ) 2 ·6H 2 O, 3.655g of Ba(NO 3 ) 2 and 10.493g of Al(NO 3 ) 3 ·6H 2 O, dissolved in 20.0mL deionized water solution, heated to 60°C in water bath and stirred to prepare solution #1; dissolved 9.906g citric acid and 2.926g ethylene glycol in 20.0mL deionized water solution, heated to 60°C in water bath and stir to prepare solution #2; mix and stir solution #1 and solution #2 in a water bath at 60°C to form a gel and dry at 105°C for 12 hours, and the dried catalyst is calcined at 700°C for 4 hours, namely The catalyst CA-1B of the present invention is obtained; the weight percentage of the catalyst in terms of oxides is as follows: the content of barium oxide is 53.6%, the content of aluminum oxide is 35.6%, and the content of cobalt oxide is 10.8%.
[0037] The CA-1B catalyst was used in the autothermal reforming reaction of acetic acid to evaluate the hydrogen production performance. / H 2 O / O 2 / N 2 =1.00 / 4.00 / 0.28 / 3.90, the conversio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com