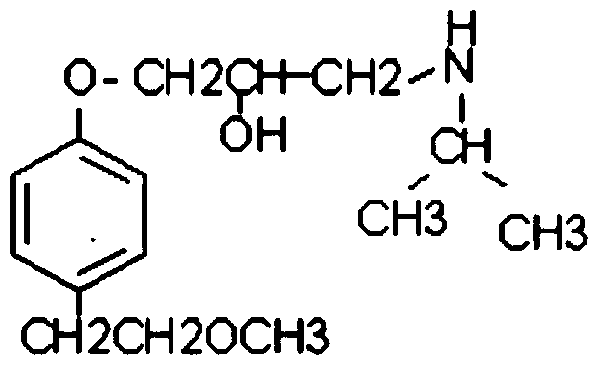
Preparation method of metoprolol intermediate
A technology of metoprolol and intermediates, applied in the field of drug synthesis, can solve the problem of high price, achieve the effect of low price, simple reaction steps, and reduce production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
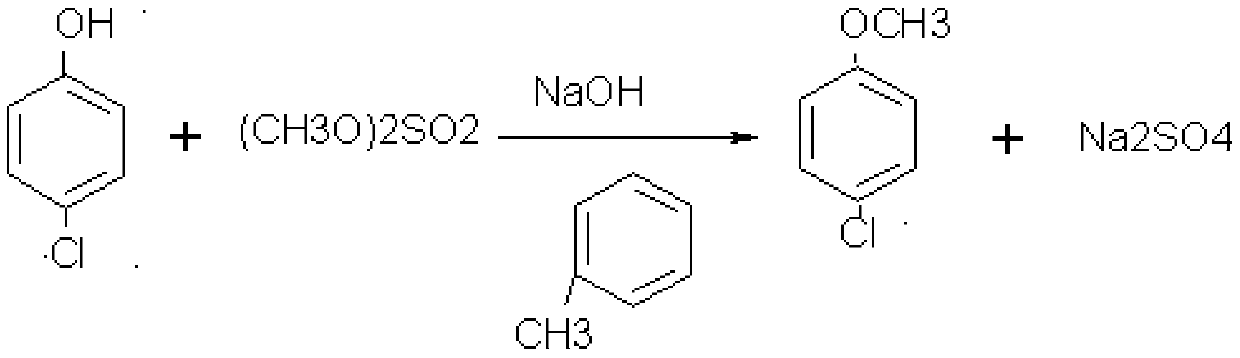
[0041] S1. Pump 500kg of toluene and 200kg of p-chlorophenol into the reaction kettle, pump in 150kg of dimethyl sulfate after completely dissolving, slowly add 150kg of caustic soda dropwise, and complete the dropwise addition in 5 hours. The reaction temperature is 40°C. After the dropwise reaction 1 hour; stand still, remove the water layer to recover sodium sulfate; after the toluene is evaporated from the organic layer, cool, add 500 kg of methyl tetrahydrofuran, dissolve, and pack into barrels to obtain 786 kg of p-chloroanisole-methyl tetrahydrofuran solution;
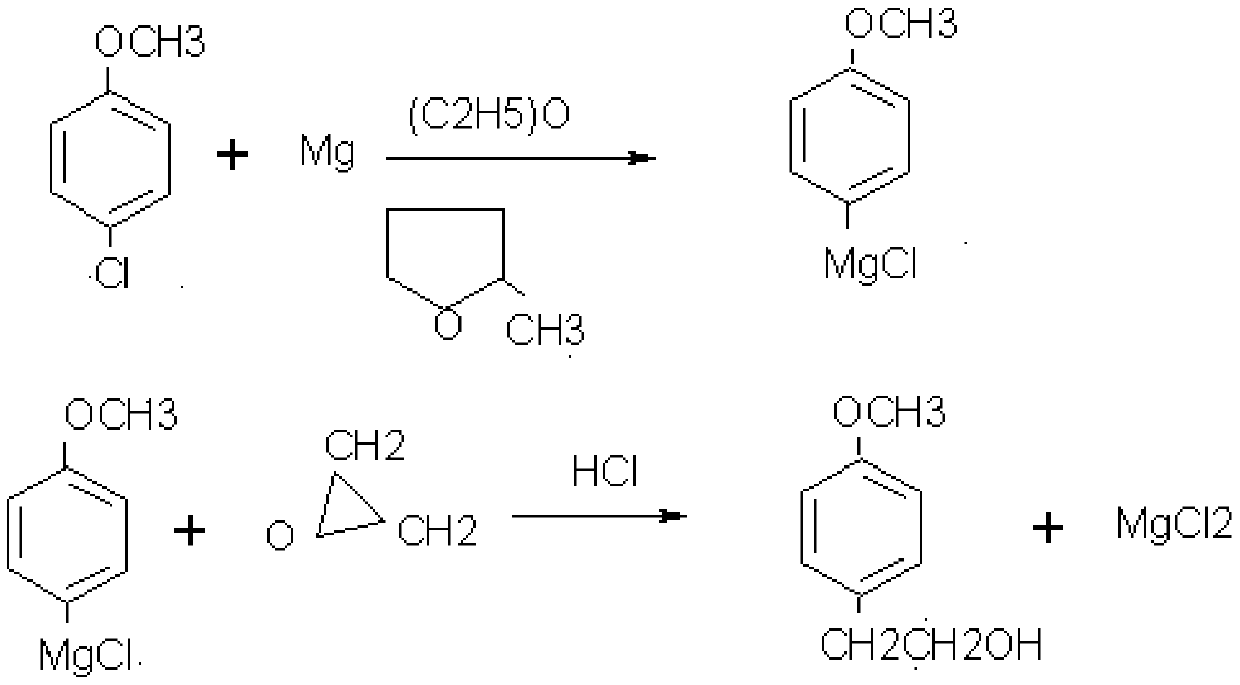
[0042] S2. Put 48kg of magnesium chips into the nitrogen-protected reaction kettle, immerse it in 600kg of ether, add p-chloroanisole-methyltetrahydrofuran solution dropwise under stirring, after the dropwise addition, react for 1h, control the reaction temperature at 15°C, and maintain the reaction Liquid steady slight reflux. Suction filter the above reaction solution into another reaction kettle, add 240kg of...
Embodiment 2
[0053] S1. Pump 500kg of toluene and 200kg of p-chlorophenol into the reaction kettle, pump in 150kg of dimethyl sulfate after completely dissolving, slowly add 150kg of caustic soda dropwise, and complete the dropwise addition in 5 hours. The reaction temperature is 40-45°C. Afterwards, react for 1 hour; let stand, remove the water layer to recover sodium sulfate; evaporate the toluene from the organic layer, cool, add 500 L of methyl tetrahydrofuran, dissolve, and put it in barrels to obtain 786 kg of p-chloroanisole-methyl tetrahydrofuran solution;
[0054] S2. Put 45kg of magnesium chips into the nitrogen-protected reaction kettle, immerse it in 600kg of ether, and add p-chloroanisole-methyl tetrahydrofuran solution dropwise under stirring. After the dropwise addition, react for 1.5h. The reaction solution was slightly refluxed steadily. Suction filter the above reaction solution to another reaction kettle, add 240kg of hydrochloric acid, under cooling condition, control t...
Embodiment 3
[0066] S1. Pump 500kg of toluene and 200kg of p-chlorophenol into the reaction kettle, pump in 150kg of dimethyl sulfate after completely dissolving, slowly add 150kg of caustic soda dropwise, and complete the dropwise addition in 5 hours. The reaction temperature is 40°C. After the dropwise reaction 1 hour; stand still, remove the water layer to recover sodium sulfate; evaporate the toluene from the organic layer, cool, add 500 L of methyl tetrahydrofuran, dissolve, put in barrels, and obtain 786 kg of p-chloroanisole-methyl tetrahydrofuran solution;
[0067] S2. Put 50kg of magnesium chips into the nitrogen-protected reaction kettle, immerse it in 600kg of ether, add p-chloroanisole-methyl tetrahydrofuran solution dropwise under stirring, after the dropwise addition, react for 1.5h, control the reaction temperature at 20°C, and maintain The reaction solution was slightly refluxed steadily. Suction filter the above reaction solution into another reaction kettle, add 240kg of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com