Thermal crosslinking functionalized triphenylamine polymer as well as preparation method and application thereof
A functionalized, thermal cross-linking technology, applied in the preparation of organic compounds, preparation of aminohydroxy compounds, chemical instruments and methods, etc., can solve the lack of purification and solubility, the limitation of colorless and transparent state, film-forming and stability problems such as poor performance, to achieve the effect of short response time, fast response rate and high transmittance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
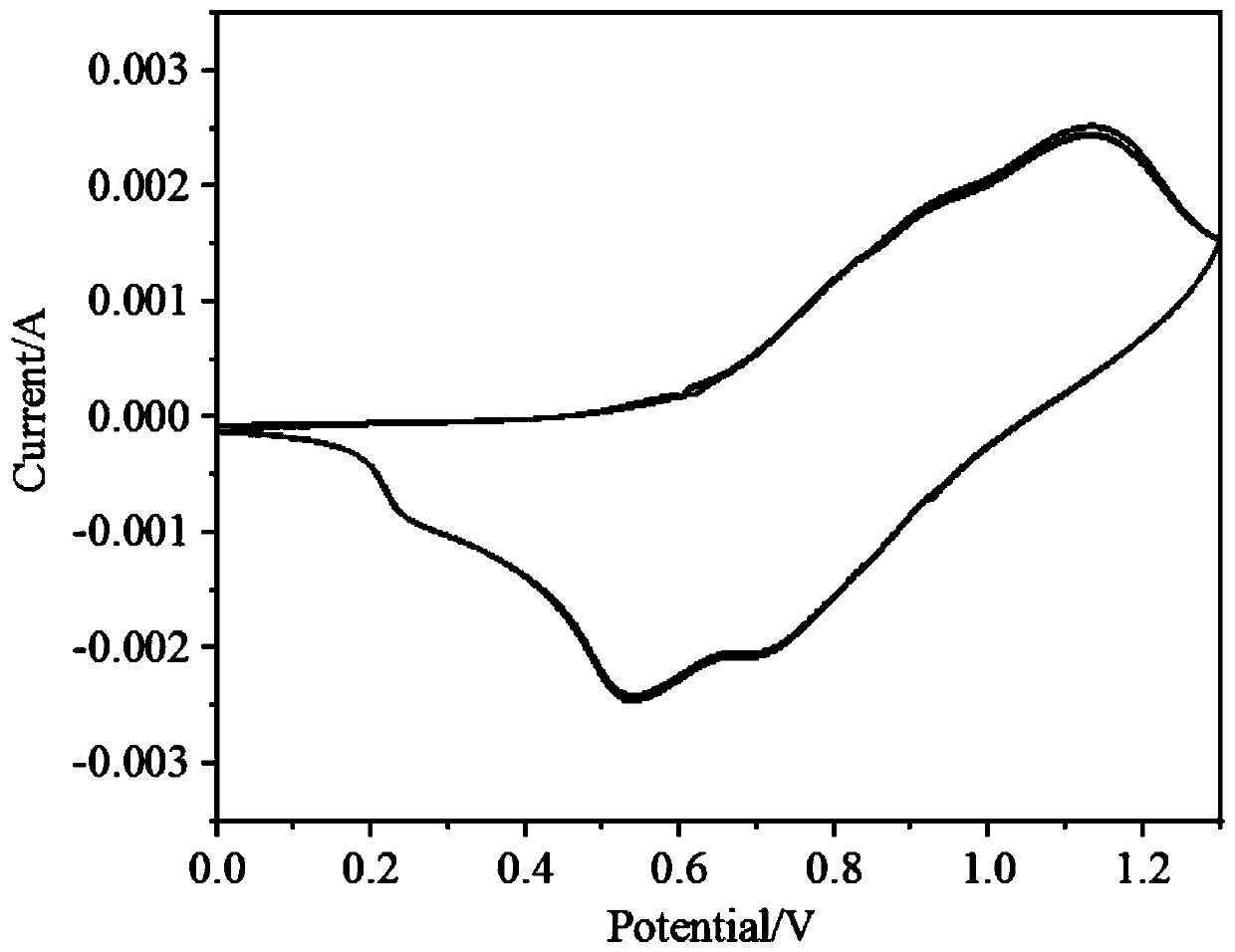
Image
Examples
Embodiment 1
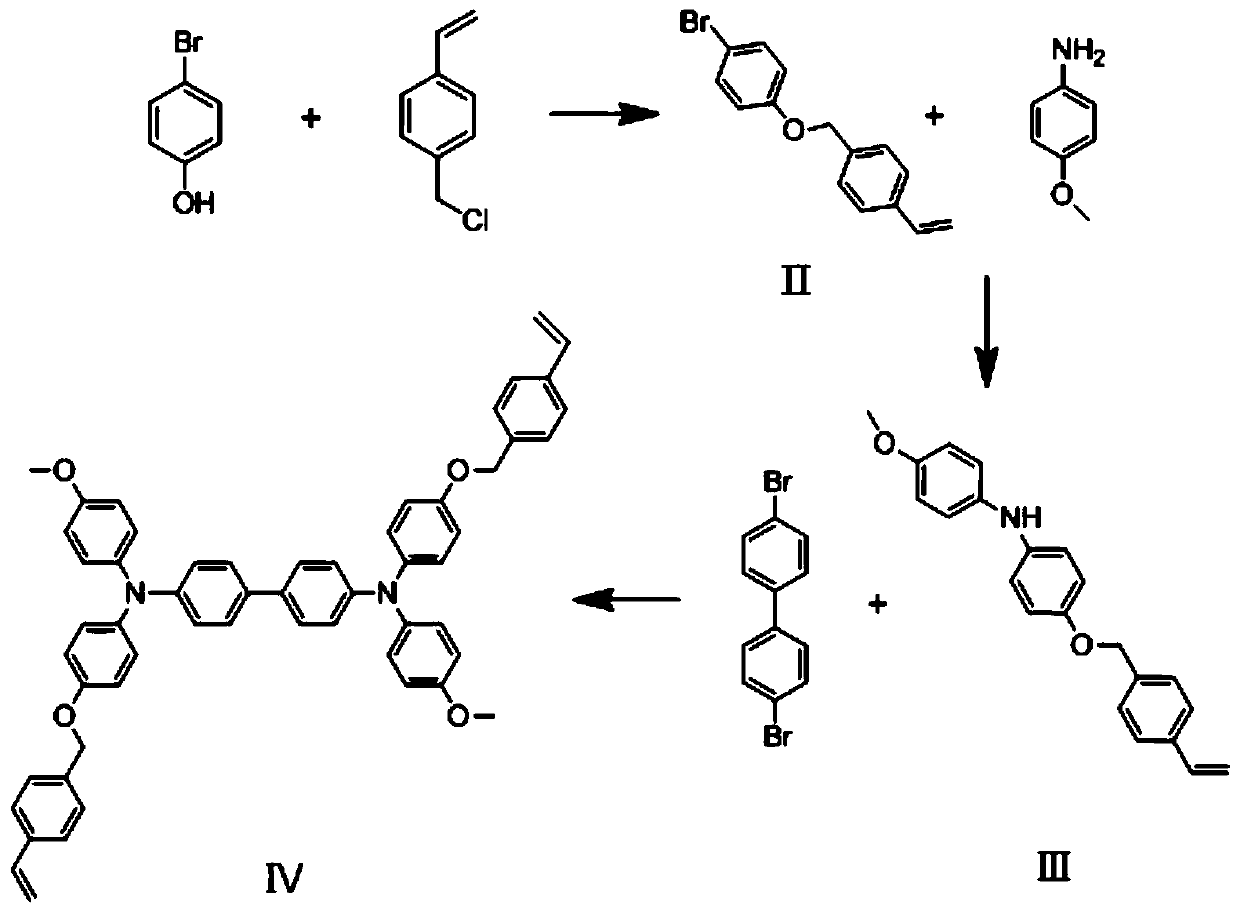
[0042] Example 1 Synthesis of 1-bromo-4-(4-vinylbenzyloxy)benzene
[0043] 4-vinylbenzyl chloride (2.2g, 14.5mmol), 4-bromophenol (2g, 14.5mmol), K 2 CO 3 (3g, 23.4mmol), 18-crown-6-ether (0.78g, 3mmol) and tetrabutylammonium iodide (0.27g, 0.8mmol) were added in 30mL of anhydrous acetone, and heated to reflux for 16h, the reaction was completed Finally, the reaction solution A was obtained. After the reaction, the reaction solution was cooled to room temperature and quenched by adding water. After that, saturated brine and dichloromethane were added for extraction, and the organic phase was collected, dried over anhydrous magnesium sulfate, concentrated under reduced pressure, and separated and purified by column chromatography. Use 300-400 mesh silica gel as the stationary phase, elute with dichloromethane / petroleum ether mixed solution with a volume ratio of 1:1 as the mobile phase, collect the eluate containing the target compound, distill off the solvent under reduced p...
Embodiment 2
[0044] Example 2 Synthesis of 4-methoxy-N-(4-(4-vinylbenzyloxy)phenylaniline
[0045] 1-Bromo-4-(4-vinylbenzyloxy)benzene (2g, 6.92mmol), p-anisidine (1.92g, 10.41mmol), KO t Bu(2.33g, 20.83mmol), Pd(OAc) 2 (0.233g, 0.67mmol), which was taken into a 100ml two-necked flask equipped with a septum. 30 mL of anhydrous toluene and tri-tert-butylphosphine (0.631 g, 2.08 mmol) were added, and the reaction mixture was refluxed under nitrogen atmosphere for 24 hours. After the reaction solution was cooled to room temperature, water was added to quench it. After that, saturated brine and dichloromethane were added for extraction, and the organic phase was collected, dried over anhydrous magnesium sulfate, concentrated under reduced pressure, and separated and purified by column chromatography. Using 300-400 mesh silica gel as the stationary phase, eluting with a dichloromethane / petroleum ether mixture with a volume ratio of 2:1 as the mobile phase, collecting the eluate containing th...
Embodiment 3
[0046] The synthesis of embodiment 3MeO-TPDSt
[0047] Dibromobiphenyl (1.43g, 3.02mmol), product (Ⅲ) 4-methoxy-N-(4-(4-vinylbenzyloxy)phenylaniline (2g, 6.04mmol), KO t Bu(1.36g, 12.08mmol), Pd(OAc) 2 (0.233g, 0.67mmol) and P(t-Bu) 3 (0.631g, 2.08mmol) was added into a two-necked flask containing 30mL of anhydrous toluene at room temperature. The reaction mixture was refluxed for 24 hours under nitrogen atmosphere. After the reaction, the reaction solution was cooled to room temperature, and quenched by adding water. After that, saturated brine and dichloromethane were added for extraction, and the organic phase was collected, dried over anhydrous magnesium sulfate, concentrated under reduced pressure, and separated and purified by column chromatography. Use 300-400 mesh silica gel as the stationary phase, and use dichloromethane / petroleum ether mixed solution with a volume ratio of 1:1 as the mobile phase for elution, collect the eluate containing the target compound, di...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com