Preparation method of ALK inhibitor Brigatinib
A technology of tini and acid application, applied in the field of medicine, can solve the problems of unsuitability for large-scale or industrialized production, low reaction yield, expensive raw materials, etc., and achieve the maximum benefit generation of industrialization, easy availability of reaction raw materials, post-processing convenient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
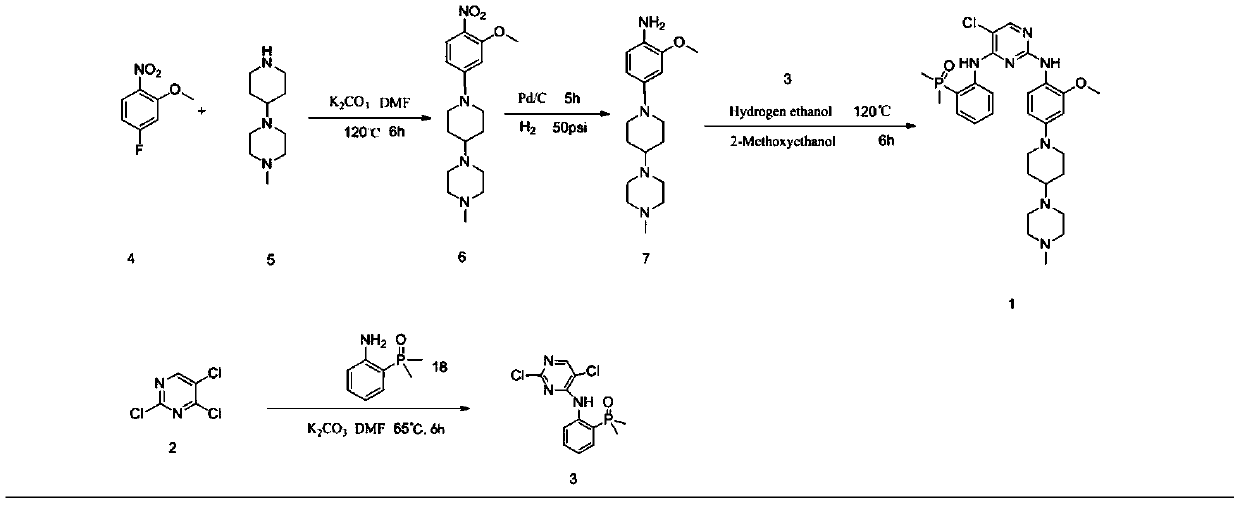
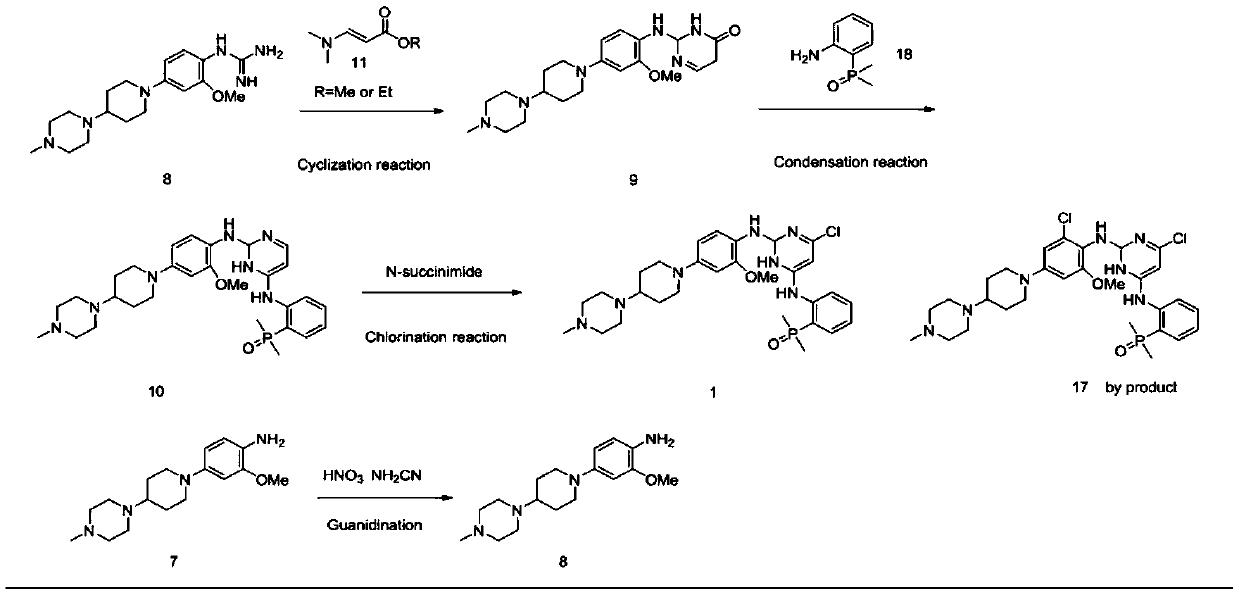
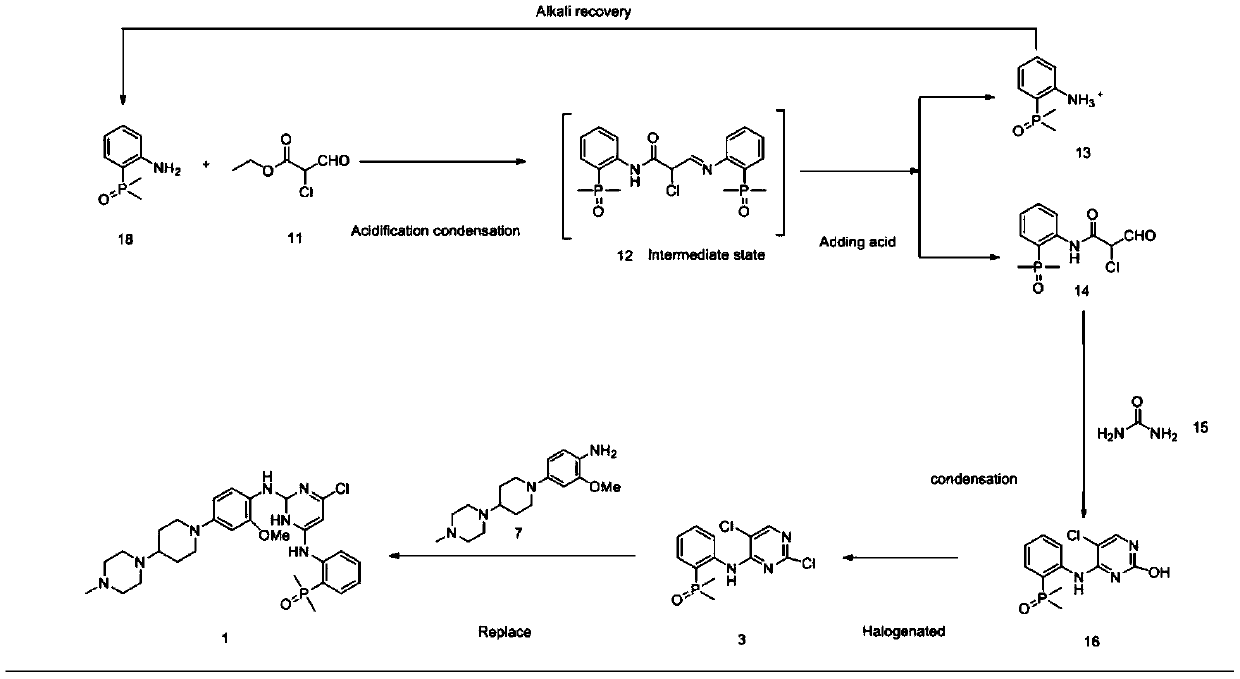
[0048] Synthesis of intermediate 1 (2,5-dichloro-N-(2-(dimethylphosphino)phenyl)pyrimidin-4-amine).
[0049]
[0050] 2,4,5-trichloropyrimidine (AP-2) (15.2g, 83mmol), 2,4,5-trichloropyrimidine and 2-(dimethylphosphoryl)aniline (AP-1) (9.4 g, 55.6 mmol, ) and K 2 HPO 4 (15.3 g, 110 mmol) in DMF (55 mL) was stirred at 65°C for 4 hours. After cooling, the reaction mixture was filtered, the filter cake was washed with ethyl acetate (20ml) and the filtrate was evaporated. The residue was dissolved in ethyl acetate (50ml), extracted three times with saturated sodium chloride solution (3×100ml), the organic matter was combined, concentrated by rotation to a yellow-white solid, and beaten three times with PE (3×20ml) to obtain the obtained The desired product 12.0g AP-3 is a yellow-white solid. Yield: 90.3%, HPLC purity >98.0%. ESI-MS(m / z): 316.0[M+H] + ,631.0[2M+H] +
Embodiment 2
[0052] Synthesis of 1-(1-(3-methoxy-4-nitrophenyl)piperidin-4-yl)4-methylpiperazine
[0053]
[0054] 2-nitro-5-fluoroanisole (17.1 g, 0.1 mol) and 1-methyl-4-(4-piperidinyl)-piperazine (18.3 g, 0.1 mol) in MeCN (65 mL) solvent , Potassium carbonate (27.6g, 0.2mol) was used as an acid application agent, stirred at reflux for 4.5 hours, cooled to room temperature and filtered with suction, and the filter cake was washed with DCM (20mL). The filtrates were combined and concentrated, the concentrated solid was dissolved in DCM (50ml), extracted three times with 1mol / L HCl solution (3×15ml), the aqueous layer was collected and adjusted to pH=8.0 with potassium carbonate, and then extracted three times with DCM (3×20ml) . It was dried over sodium sulfate, filtered, and concentrated in vacuo to obtain the desired product AP-6 as 29.34 g of bright yellow powder, with a yield of 87.8% and a purity of >99.8% by HPLC. ESI-MS (m / z): 335.2[M+H]+, 669.4[2M+H]+
Embodiment 3
[0056] Synthesis of Intermediate 2 (2-methoxy-4-[4-(4-methyl-1-piperazinyl)-1-piperazinyl]-aniline)
[0057]
[0058] Dissolve 1-(1-(3-methoxy-4-nitrophenyl)piperidin-4-yl)4-methylpiperazine (AP-6) (20 g, 0.06 mol) in EtOH (800 mL) After neutralization, catalytic hydrogenation (10 psi H 2 ) 2.5 hours. The mixture was filtered through celite and the filtrate was concentrated to give a purple solid. Yield: 17.3 g, 95% yield, >99.8% purity. ESI-MS(m / z):305.2[M+H]+609.4[2M+H]+
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com