Active immune regulation particle, and preparation method and application thereof
An active immunization and microparticle technology, applied in chemical instruments and methods, pharmaceutical formulations, vertebrate antigen components, etc., can solve the problems of poor anti-tumor effect of vaccines, break immune tolerance, promote amplification, and improve immune response Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
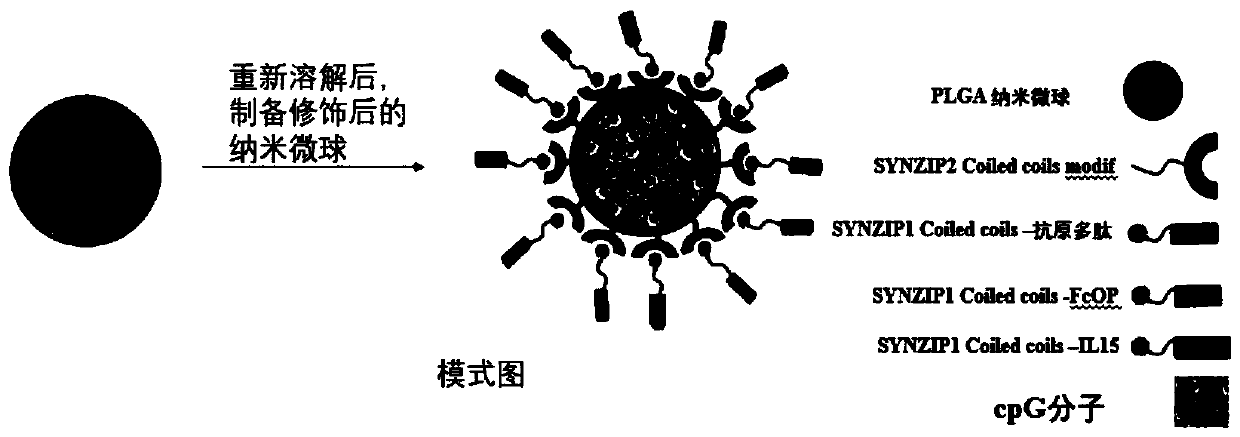
[0062] The preparation method of the active immune regulation particles of the present invention at least includes the following steps:
[0063] 1) preparing SYNZIP2-CpG-microcarrier particles;
[0064] 2) Connect SYNZIP1 to T cell promoter, antibody Fc fragment and antigen respectively to obtain connector I, connector II and connector III;
[0065] 3) Mix linker I, linker II and linker III with SYNZIP2-CpG-microcarrier particles to obtain the active immune regulation particles.
[0066] The preparation method of described SYNZIP2-CpG-microcarrier particle comprises the steps:
[0067] 1) Mixing SYNZIP2 with an activator for activation; mixing the activated SYNZIP2 with a cross-linking agent for a cross-linking activation reaction to obtain a mixture I;
[0068] 2) activating by mixing the nucleic acid molecule CpG with an activator; mixing the activated nucleic acid molecule CpG with a cross-linking agent to obtain a mixture II;
[0069] 3) Dissolving the microcarrier in a...
Embodiment 1
[0083] Example 1 Construction process of active immune regulation microparticle assembly
[0084] 1. SYNZIP-1-FcOP protein
[0085] SYNZIP-1-FcOP protein is obtained by gene synthesis. Specifically, get:
[0086]
[0087]
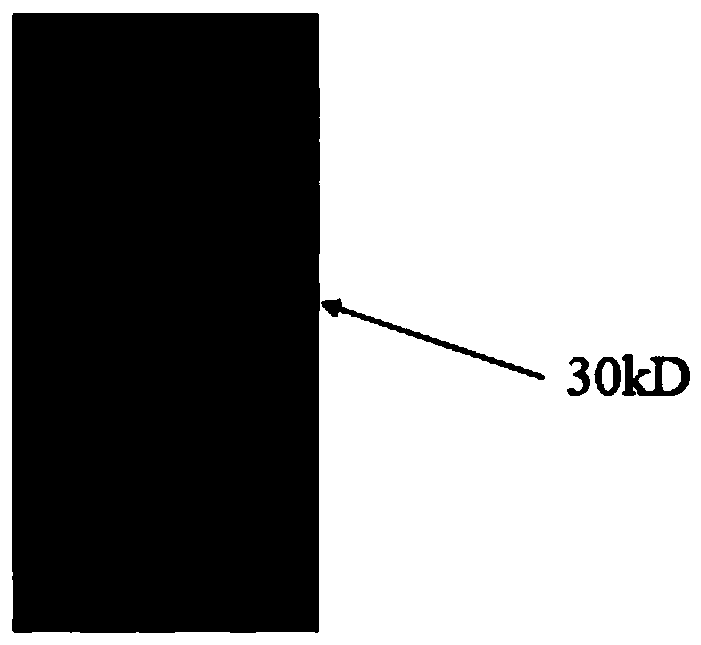
[0088] SYNZIP-1 -linker-FcOP amino acid sequence (30KD)
[0089] MEFGLSWLFLVAILKGVQCNLVAQLENEVASLENENETLKKKNLHKKDLIAYLEKEIANLRKKIEE (GGGGS) 3 PELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNATYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIAATISKAKGQPREPQVYTLPPSRDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFALYCSLTVNDKSLHFSG
[0090] (SEQ ID NO: 12)
[0091] 2. Cloning of IL-15-SYNZIP1 gene
[0092] Restriction sites EcoR I and Not I were designed at both ends of IL-15-SYNZIP1, and Suzhou Jinweizhi Biotechnology Co., Ltd. was commissioned to synthesize them by whole-gene synthesis to obtain the PUC19-IL-15-SYNZIP1 cloning plasmid.
[0093] The obtained PUC19-IL-15-SYNZIP1 cloning plasmid and PTT5 plasmid were respectively digested wit...
Embodiment 2
[0107] Example 2 Preparation of Active Immunomodulatory Particles
[0108] 1. Preparation of PLGA nanospheres
[0109] 20mg SYNZIP2 (sulfo-SMCC) cross-linking activation reaction: first, SYNZIP2 activation, dissolve SYNZIP2 with 10ml PBS, add TCEP, open disulfide bond; dialyze to remove TCEP, and get activated SYNZIP2. Then add sulfo-SMCC, dialyze to remove excess sulfo-SMCC after cross-linking, and obtain 20 mg of SYNZIP2-sulfo-SMCC solid powder after freeze-drying.
[0110] (1) Cross-linking reaction of nucleic acid molecule CpG (CpG ODN 2006 and ODN M362) 10 mg with sulfo-SMCC: First, CpG is activated, CpG is dissolved with WFI, TCEP is added to open the disulfide bond; then TCEP is removed by desalting to obtain activated CpG .
[0111] Then add sulfo-SMCC, after cross-linking, alcohol sedimentation and centrifugation to obtain solid CpG-sulfo-SMCC.
[0112] The sequence of CpG ODN 2006 is (TCGTCGTTTTGTCGTTTTGTCGTT) (SEQ ID NO: 19)
[0113] The sequence of CpG-SH ODN M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com