Fatty acyl amino acid polyhydroxy ester compound and preparation method thereof
A fatty acylamino acid, polyhydroxy compound technology, applied in the field of organic compound synthesis, can solve the problems that have not yet been seen, and achieve the effects of excellent low irritation, unique skin feel, and good affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
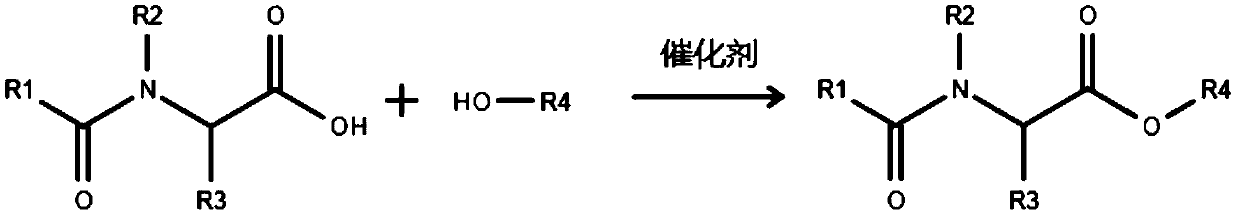
[0034] A preparation method of the above-mentioned fatty acylamino acid polyhydroxy ester compound, comprising the following steps:
[0035] (1) Take the fatty acylamino acid and polyhydroxy compound in the formula amount, add the catalyst, put it in a vacuum environment, heat it to 120-140°C while stirring, and keep it warm for 2-6h;
[0036] (2) Warm up the mixture obtained in step (1) to 160-200° C., keep it warm for 15-60 minutes, and cool to room temperature;
[0037] (3) Add activated carbon to the substance obtained in step (2), and filter to obtain the fatty acylamino acid polyhydroxy ester compound.
[0038]The addition amount of the catalyst in the reaction system is 0.1-5% of the total mass of the fatty acyl amino acid and the polyhydroxy compound.
[0039] As a further preferred solution, the vacuum degree of the vacuum environment in step (1) is -0.095MPa; the temperature in step (1) is 145°C, and the holding time is 3h; the temperature in step (2) is 160°C, and ...
Embodiment 1
[0041] A fatty acylamino acid polyhydroxy ester compound prepared by the following method:
[0042] (1) Take 30g of lauroyl sarcosine and 20g of xylitol, add 0.05g of catalyst p-toluenesulfonic acid, place in a vacuum of -0.095MPa, heat to 145°C while stirring, and keep warm for 3h;
[0043] (2) The mixture obtained in step (1) is heated up to 160° C., and after being incubated for 15 minutes, the heating is stopped and cooled to room temperature;
[0044] (3) Add activated carbon to the substance obtained in step (2) for decolorization, and obtain the fatty acylamino acid polyhydroxy ester compound of this embodiment after filtration.
Embodiment 2
[0046] A fatty acylamino acid polyhydroxy ester compound prepared by the following method:
[0047] (1) Take 25g of palmitoyl sarcosine and 20g of propylene glycol, add 0.05g of catalytic phosphoric acid, place in a vacuum of -0.095MPa, heat to 145°C while stirring, and keep warm for 3h;
[0048] (2) The mixture obtained in step (1) is heated up to 160° C., and after being incubated for 30 minutes, the heating is stopped and cooled to room temperature;
[0049] (3) Add activated carbon to the substance obtained in step (2) for decolorization, and obtain the fatty acylamino acid polyhydroxy ester compound of this embodiment after filtration.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com