Nucleic acid releasing agent and HPV virus nucleic acid detection kit
A nucleic acid release agent, nucleic acid technology, applied in the biological field, can solve the problem of inability to accurately quantify HPV DNA, and achieve the effects of increased experimental cost, high surface tension, and rapid lysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
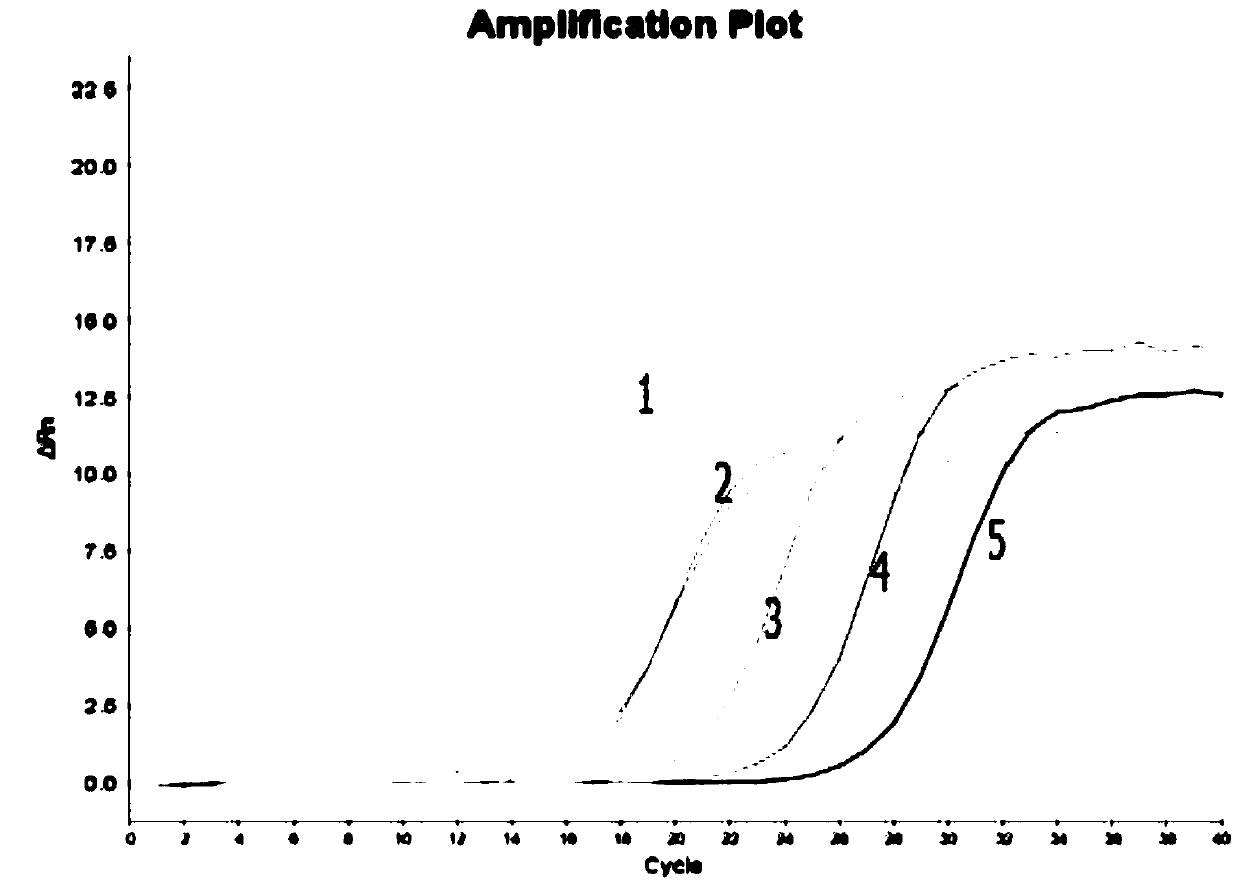
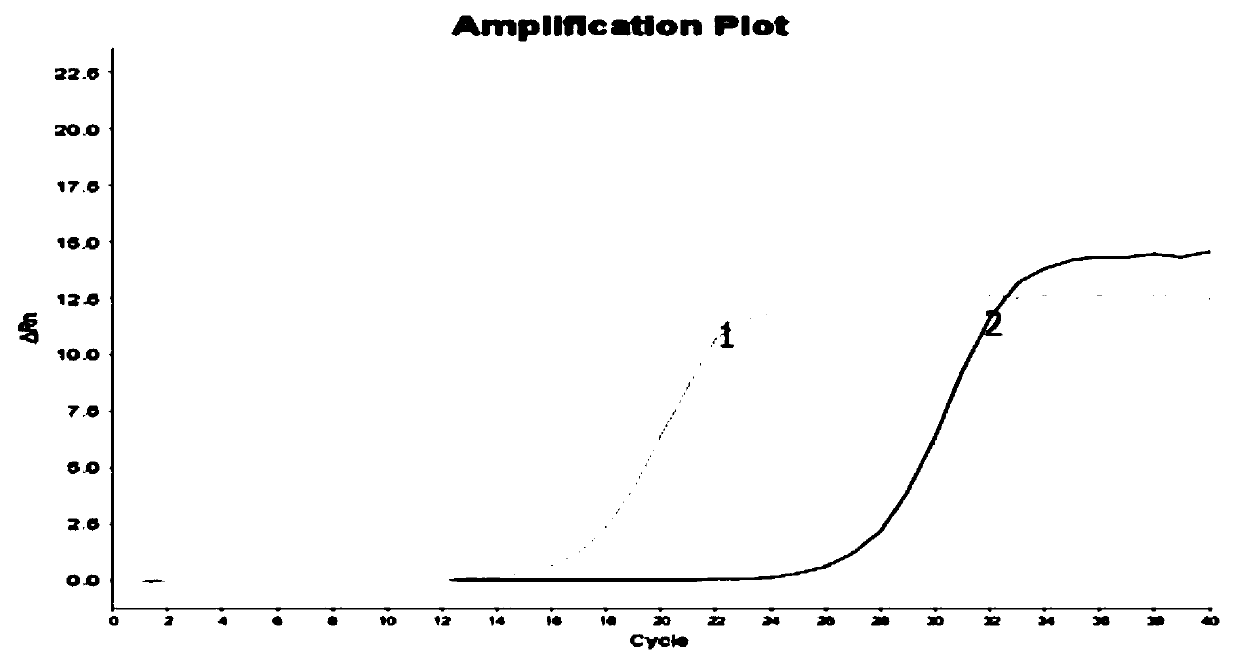
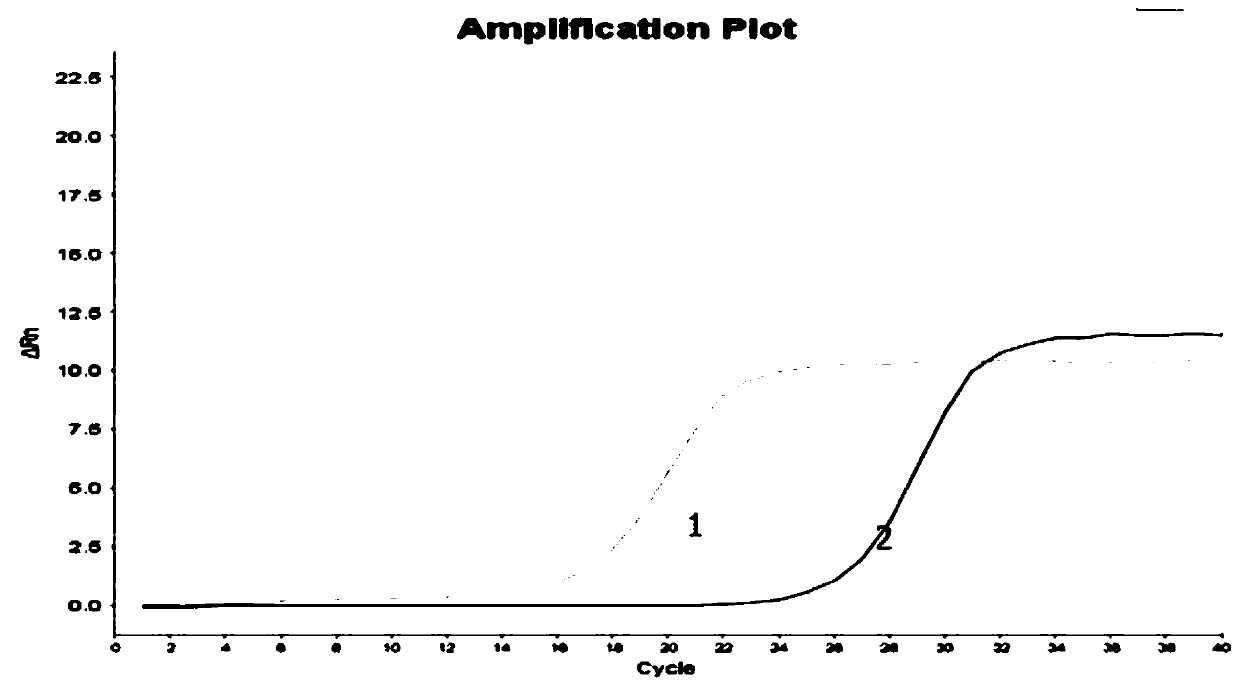
Image
Examples
Embodiment 1
[0036] A nucleic acid releasing agent, comprising the composition of 5wt.% Triton X-100 and DCDE, the sodium hydroxide of 50mmol / L, the ethanol of 0.2Vol.%, the sodium dodecylsulfonate of 1wt.%, balance for water. The mass ratio of Tritonx-100 and DCDE is 1:3.
Embodiment 2
[0038]A nucleic acid releasing agent, comprising the composition of 10wt.% Triton X-100 and DCDE, the sodium hydroxide of 100mmol / L, the ethanol of 0.8Vol.%, the sodium dodecylsulfonate of 0.5wt.%, remaining The amount is water. The mass ratio of Tritonx-100 and DCDE is 1:2.
Embodiment 3
[0040] A nucleic acid releasing agent, comprising the composition of 20wt.% Triton X-100 and DCDE, the sodium hydroxide of 200mmol / L, the ethanol of 1Vol.%, the sodium dodecylsulfonate of 0.8wt.%, balance for water. The mass ratio of Tritonx-100 and DCDE is 1:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com