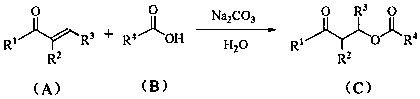Novel synthesis method of ester compound
A technology for ester compounds and a synthesis method, which is applied in the field of ester compound synthesis, can solve the problems of narrow substrate range and low reaction yield, and achieves the effects of environmental friendliness, simple reaction operation and high atom utilization rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020]
[0021] Add Na to a 10 mL single-necked small flask 2 CO 3 Aqueous solution (0.2 M, 0.5 mL), then added phenyl vinyl ketone (132 mg, 1.0 mmol) and acetic acid (120 mg, 2.0 mmol) in sequence, stirred and reacted at room temperature for 12 h, detected the reaction by TLC plate, and the reaction of raw materials was complete . Then add 30 ml of ethyl acetate, wash with water successively, wash with saturated brine, wash with anhydrous Na 2 SO 4 It was dried, filtered, concentrated under reduced pressure, and separated by column chromatography to obtain compound 1 (175 mg, 91%).
[0022] White solid, melting point 54-55 o C; 1 H NMR (400 MHz, CDCl 3 ): δ 7.96 (d, J = 7.6 Hz,2H), 7.57 (d, J = 7.2 Hz, 1H), 7.48 (t, J = 7.6 Hz, 2H), 4.52 (t, J = 6.3 Hz,2H), 3.32 (t, J = 6.3 Hz, 2H), 2.03 (s, 3H); 13 C NMR (100 MHz, CDCl 3 ): δ196.97, 170.97, 136.52, 133.39, 128.66, 128.02, 59.61, 37.28, 20.86.
Embodiment 2
[0024]
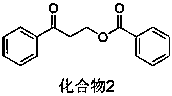
[0025] Add Na to a 10 mL single-necked small flask 2 CO 3 Aqueous solution (0.2 M, 0.5 mL), then add phenyl vinyl ketone (132 mg, 1.0 mmol) and benzoic acid (244 mg, 2.0 mmol) successively, stir the reaction at room temperature for 12 h, TLC plate detection reaction, raw material reaction completely. Then add 30 ml of ethyl acetate, wash with water successively, wash with saturated brine, wash with anhydrous Na 2 SO 4 It was dried, filtered, concentrated under reduced pressure, and separated by column chromatography to obtain compound 2 (163 mg, 64%).
[0026] White solid; melting point 55-56 o C; 1 H NMR (400 MHz, CDCl 3 ): δ 7.99 (d, J = 8.2Hz, 4H), 7.65 – 7.36 (m, 6H), 4.78 (t, J = 6.4 Hz, 2H), 3.45 (t, J = 6.4 Hz,2H); 13 C NMR (100 MHz, CDCl 3 ): δ197.12, 166.57, 136.66, 133.47, 133.04, 130.06, 129.65, 128.76, 128.37, 128.16, 60.35, 37.57.
Embodiment 3
[0028]
[0029] Add Na to a 10 mL single-necked small flask 2 CO 3 Aqueous solution (0.2 M, 0.5 mL), then add phenyl vinyl ketone (132 mg, 1.0 mmol) and cinnamic acid (296 mg, 2.0 mmol) successively, stir the reaction at room temperature for 12 h, TLC plate detection reaction, raw material reaction completely. Then add 30 ml of ethyl acetate, wash with water successively, wash with saturated brine, wash with anhydrous Na 2 SO 4 It was dried, filtered, concentrated under reduced pressure, and separated by column chromatography to obtain compound 3 (202 mg, 72%).
[0030] White solid, melting point 70-71 ºC; IR (KBr, cm -1 ) υ 3401, 2977, 1709, 1633, 1337, 1182, 996, 747, 688, 487; 1 H NMR (400 MHz, CDCl 3 ) δ 7.98 (d, J = 8.0 Hz, 2H), 7.66 (d, J = 16.0 Hz, 1H), 7.56 (d, J = 4.0 Hz, 1H), 7.48 (d, J= 8.0 Hz,4H), 7.36 (s, 3H), 6.41 (d, J = 16.0 Hz, 1H), 4.66 (t, J = 6.0 Hz, 2H), 3.39(t, J = 6.0 Hz, 2H). 13 C NMR (100 MHz, CDCl 3 ) δ 197.06, 166.82, 145.03,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com