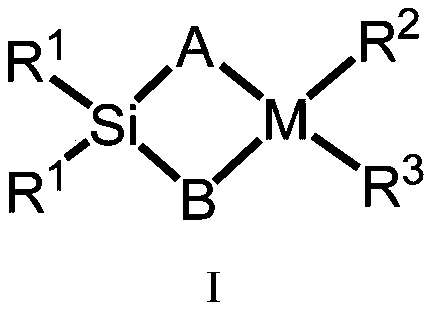
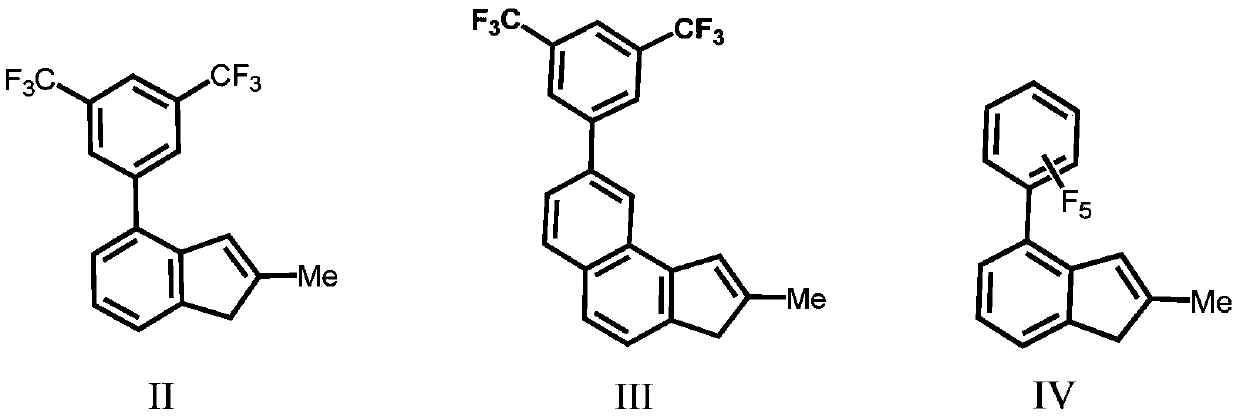
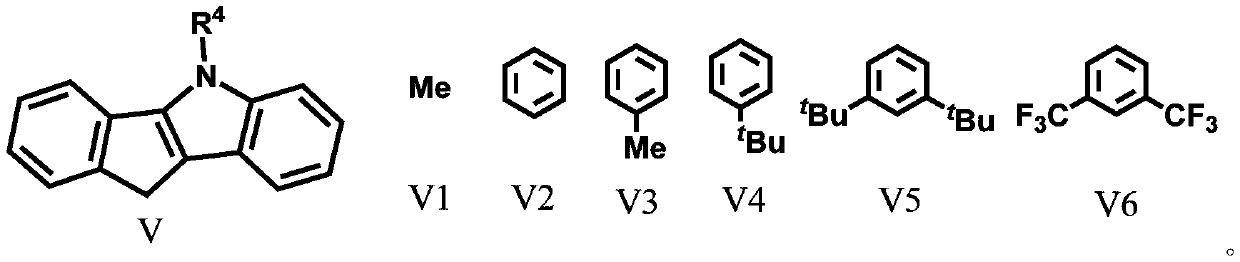
Silicon-bridged metallocene complexes containing indenoindole structure and application of silicon-bridged metallocene complexes
A technology of metallocene complexes and indenoindole structures, which is applied in the field of preparation of olefin polymerization catalysts, can solve the problem of low isotacticity of polypropylene, and achieve the effects of simple preparation, broad application prospects and good controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Ligand L1-1(2-methyl-4-3,5-bistrifluoromethyl-phenyl-indene)(N-methyl-5,10-hydrogen-indenoindole)dimethylsilyl preparation
[0044] Under a nitrogen atmosphere, 2-methyl-4-3,5-bistrifluoromethyl-phenyl-indene (3.42g / 10mmol) was dissolved in 50mL of anhydrous tetrahydrofuran, and n-butyl was slowly added at -78°C Lithium (2.5M, 4mL / 10mmol), then slowly rise to room temperature and continue to stir the reaction for 12 hours, add the lithium salt solution generated by the reaction at -78°C in the tetrahydrofuran solution of dimethyldichlorosilane (50mmol), gradually Stirring was continued for 12 hours after warming to room temperature. The solvent and excess dimethyldichlorosilane were removed under reduced pressure, and 50 mL of anhydrous tetrahydrofuran was added to the system. Meanwhile, the compound N-methyl-5,10-hydro-indenoindole (2.19 g / 10 mmol) was treated with n-butyllithium (2.5 M, 4 mL / 10 mmol) in the same manner at -78 °C Indenyllithium was generated, and th...
Embodiment 2
[0046] Ligand L1-2(2-methyl-4-3,5-bistrifluoromethyl-phenyl-indene)(N-phenyl-5,10-hydrogen-indenoindole)dimethylsilyl preparation
[0047] The experimental procedure was the same as in Example 1, and 5.78 g, 8.5 mmol of ligand L1-2 was obtained, yield: 85%. Anal. Calcd for C 41 h 31 f 6 NSi: C, 72.44; H, 4.60; N, 2.06. Found: C, 68.72; H, 5.12; N, 1.72.
Embodiment 3
[0049] Ligand L1-3(2-methyl-4-3,5-ditrifluoromethyl-phenyl-indene)(N-p-methylphenyl-5,10-hydrogen-indenoindole)dimethyl Preparation of base silicon
[0050] The experimental procedure was the same as in Example 1, and 6.04 g, 8.7 mmol of ligand L1-3 was obtained, yield: 87%. Anal. Calcd for C 42 h 33 f 6 NSi: C, 72.71; H, 4.79; N, 2.02. Found: C, 69.16; H, 5.18; N, 1.85.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com