New application of laxative formula using five fruits and vegetables as raw materials and being capable of increasing intestinal mucus layer and protecting intestinal tract
A new application, intestinal technology, applied in the direction of plant raw materials, medical raw materials derived from fungi, medical preparations containing active ingredients, etc., can solve problems such as unsuitable constipation, severe constipation, and damage to the bacterial flora structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1 Goblet cell proliferation pharmacological experiment
[0064] 1. Grouping, feeding and administration of rats
[0065] The SD rats were adaptively fed for 1 week, weighed, and randomly distributed into 5 groups (n=10) according to body weight. The model group and the treatment group were given loperamide (LOP) by subcutaneous injection for 2 weeks to induce the rat constipation model; from the second week onwards, the positive drug Maren Capsule (MRC) and Modification Capsules (TTC), for 1 week. TTC and MRC were suspended in 0.3% sodium carboxymethylcellulose (CMC-Na) solution. The specific modeling and treatment methods are as follows:
[0066] Group 1: normal group (NC), subcutaneous injection of normal saline (10ml / kg), given 0.3% CMC-Na (10mL / kg / d).
[0067] Group 2: model group (CP), LOP (10ml / kg), given 0.3% CMC-Na (10mL / kg / d).
[0068] Group 3: Positive drug group (MRC), subcutaneous injection of LOP (10ml / kg), given MRC (126mg / kg / d).
[0069] ...
Embodiment 2
[0074] Embodiment 2 mucus layer pharmacological experiment
[0075] Methods for the detection of colonic mucus
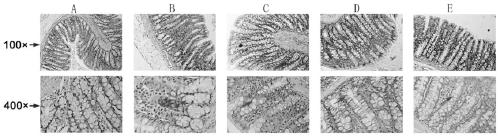
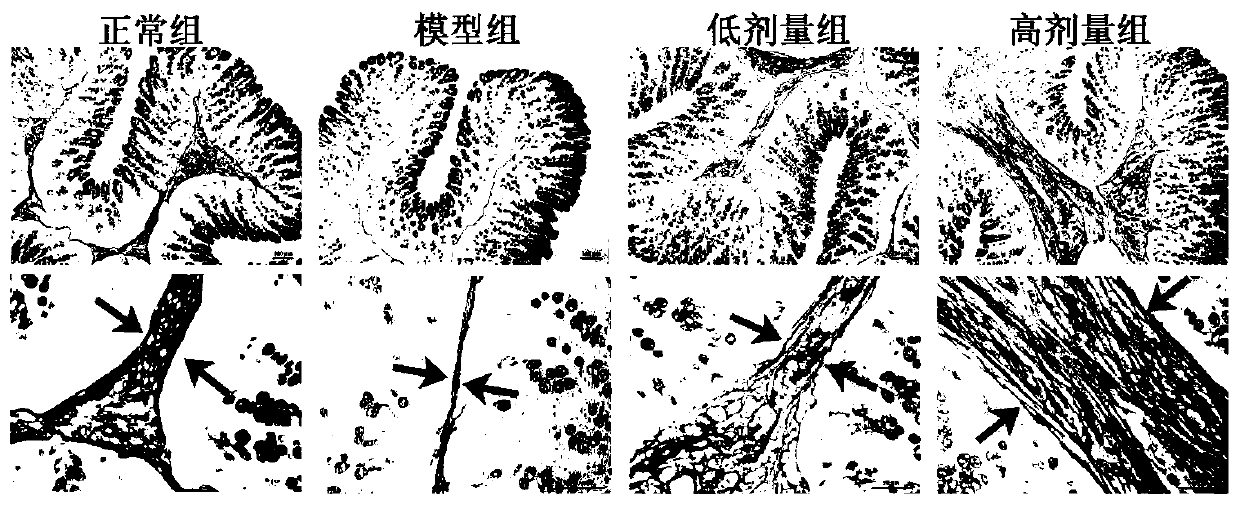
[0076] Tissue samples: After 3 weeks of the experiment, the rats were fasted for 12 hours, weighed, and then anesthetized with 10% chloral hydrate (0.3mL / 100g), and the colon tissues were immediately removed, fixed in 10% buffered formalin for 48 hours and wrapped Embedded in paraffin, cut into 5 μm thick sections and stained with Alcian blue to assess the thickness of the colonic mucus layer.
[0077] Colonic mucus test results
[0078] The study found that the adherent rectal mucus layer was significantly reduced in patients with chronic constipation. The use of lubiprostone can increase the secretion of mucus and mucin. Therefore, constipation may be related to the secretion of mucus and mucin. In this experiment, in order to verify the correlation between constipation and mucus, we detected the thickness of the mucus layer in the colon tissue ( figure 2 )....
Embodiment 3
[0079] Embodiment 3 clinical trial 1
[0080] Gastric ulcer is a common digestive system disease in clinical practice, and the main patients are mostly the elderly. The clinical symptoms in the early stage are not obvious. When the gastric ulcer is severe, the main symptom is perforation or bleeding. If you do not seek medical treatment in time, it will seriously affect your daily life and quality of life, and eventually lead to the appearance of cancer. Our company analyzed and summarized the clinical data of 89 patients with gastric ulcer from May 2018 to October 2018. The report is as follows:
[0081] 1. Patient information: 62 males and 27 females, aged 40-65 years, average age (53.6±2.8) years; body weight 44-75kg, average (57.3±3.8) kg; course of disease 0.5-15 years. The main clinical manifestations are stomach pain with a sense of bloating, loss of appetite, and lack of energy. Nausea, nausea, vomiting, acid reflux, belching, poor daily bowel movements, melena and o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com