Method for preparing low-carbon olefin from synthesis gas
A low-carbon olefin and synthesis gas technology, which is applied in the field of co-production of gasoline and synthesis gas to produce low-carbon olefins, can solve the problems of large energy consumption, low selectivity of low-carbon olefins, low catalyst activity, etc., and achieve high conversion rate and methane The effect of low selectivity and simple separation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
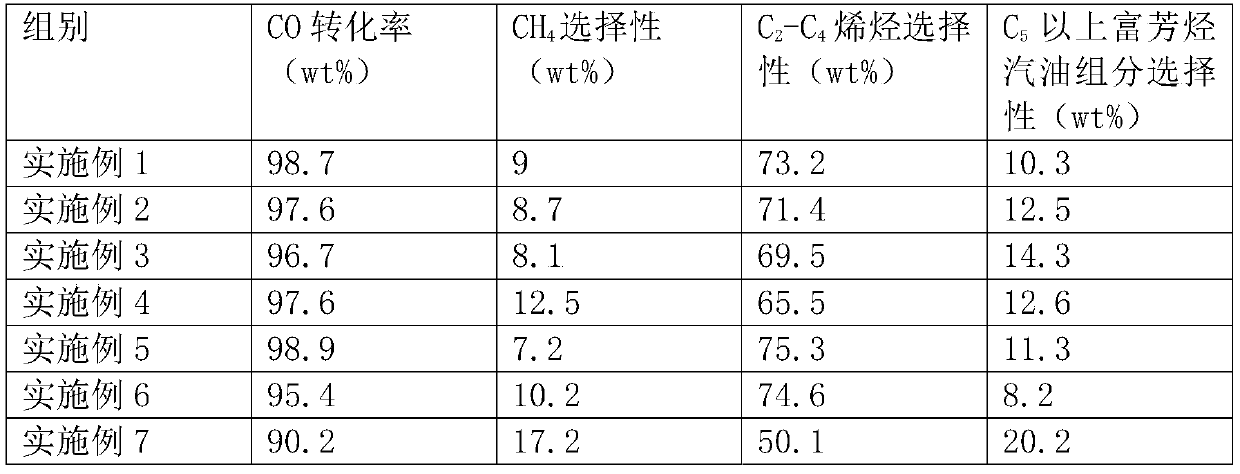
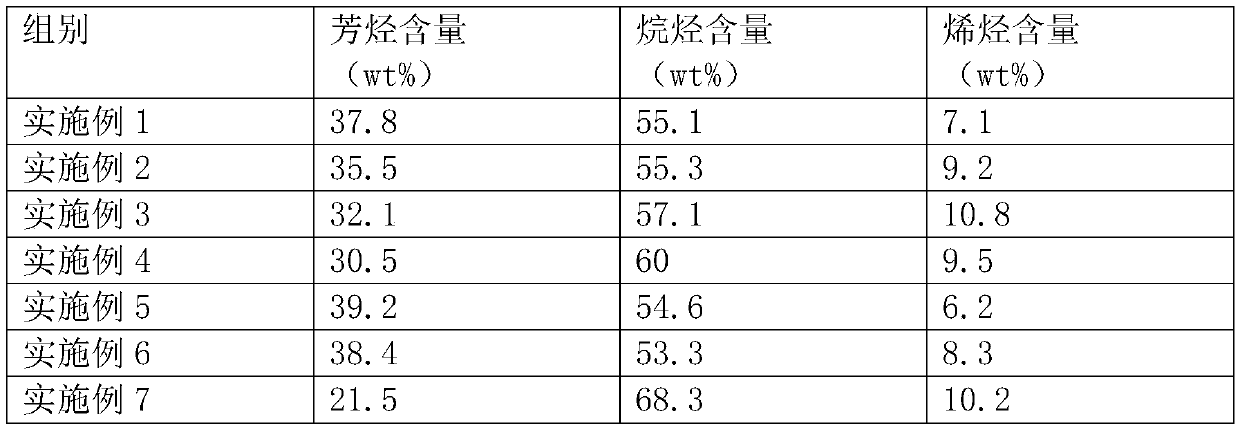
Embodiment 1
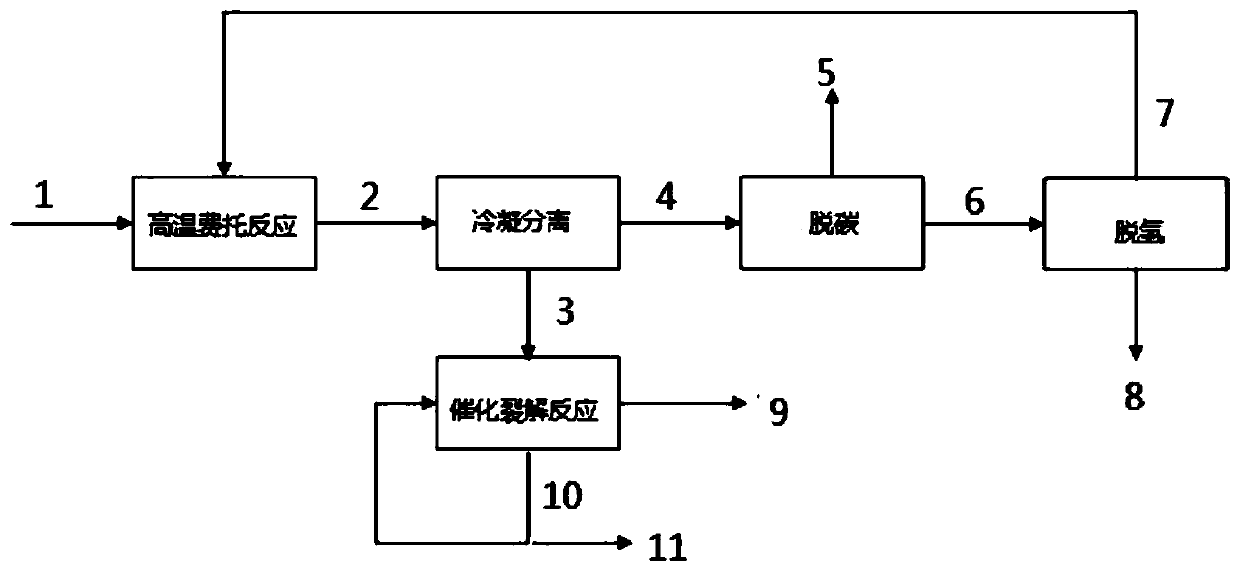
[0053] use as figure 1 The process described in the example verification process uses a fluidized bed reactor for the high-temperature Fischer-Tropsch reaction process, and a fixed-bed reactor for the catalytic cracking reaction process.
[0054] High temperature Fischer-Tropsch reaction conditions: H 2 / CO=1 / 1 (molar ratio, the same below), reaction temperature 340°C, reaction pressure 2MPa, catalyst load (reaction actual volume space velocity) 8000h -1 , 80% carbon monoxide, hydrogen recycle stream ( figure 1 The middle stream 7) is circulated back to the high temperature Fischer-Tropsch reaction zone.
[0055] High temperature Fischer-Tropsch uses Fe catalyst 100 Zn 35 Cr 15 Mg 60 Al 120 Na 3 o x .
[0056] Catalytic cracking reaction conditions: reaction temperature 500°C, reaction pressure, 0.1MPa, catalyst load (reaction actual mass space velocity) 20h -1 , 80% of the recycle stream of aromatics-rich oil with carbon five or more is recycled back to the catalyt...
Embodiment 2
[0062] use as figure 1 The process described in the example verification process uses a fluidized bed reactor for the high-temperature Fischer-Tropsch reaction process, and a fixed-bed reactor for the catalytic cracking reaction process.
[0063] High temperature Fischer-Tropsch reaction conditions: H 2 / CO=1.5 / 1, reaction temperature 320°C, reaction pressure 1.5MPa, catalyst load (reaction actual volume space velocity) 4000h -1 , 90% of the carbon monoxide and hydrogen circulating streams are recycled back to the high-temperature Fischer-Tropsch reaction zone.
[0064] High temperature Fischer-Tropsch uses Fe catalyst 100 Zn 50 Cr 12 Mg 30 Al 90 Na 8 o x .
[0065] Catalytic cracking reaction conditions: reaction temperature 600°C, reaction pressure, 0.5MPa, catalyst load (reaction actual mass space velocity) 30h -1 , 90% of the recycle stream of aromatics-rich oil with carbon five or more is recycled back to the catalytic cracking reaction zone.
[0066] Catalytic...
Embodiment 3
[0071] use as figure 1 The process described in the example verification process uses a fluidized bed reactor for the high-temperature Fischer-Tropsch reaction process, and a fixed-bed reactor for the catalytic cracking reaction process.
[0072] High temperature Fischer-Tropsch reaction conditions: H 2 / CO=1.5 / 1, reaction temperature 350°C, reaction pressure 2.5MPa, catalyst load (reaction actual volume space velocity) 20000h -1 , 100% carbon monoxide and hydrogen recycle streams are recycled back to the high temperature Fischer-Tropsch reaction zone.
[0073] High temperature Fischer-Tropsch uses Fe catalyst 100 mn 75 Cr 15 Mg 60 Al 120 K 3 o x .
[0074] Catalytic cracking reaction conditions: reaction temperature 550°C, reaction pressure, 0.3MPa, catalyst load (reaction actual mass space velocity) 30h -1 , 100% of the recycle stream of rich aromatics oil with carbon five or more is recycled back to the catalytic cracking reaction zone.
[0075] Catalytic crackin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com