Biochar electrochemical reforming hydrogen production method based on multi-stage utilization of biomass
A technology for reforming hydrogen and biochar, applied in the direction of electrodes, electrolysis components, electrolysis process, etc., can solve the problems of electrocatalyst source and cost limitation, slow oxidation rate, etc., achieve high efficiency and low cost hydrogen production, high and low Effect of carbon emission and promotion of oxidation reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
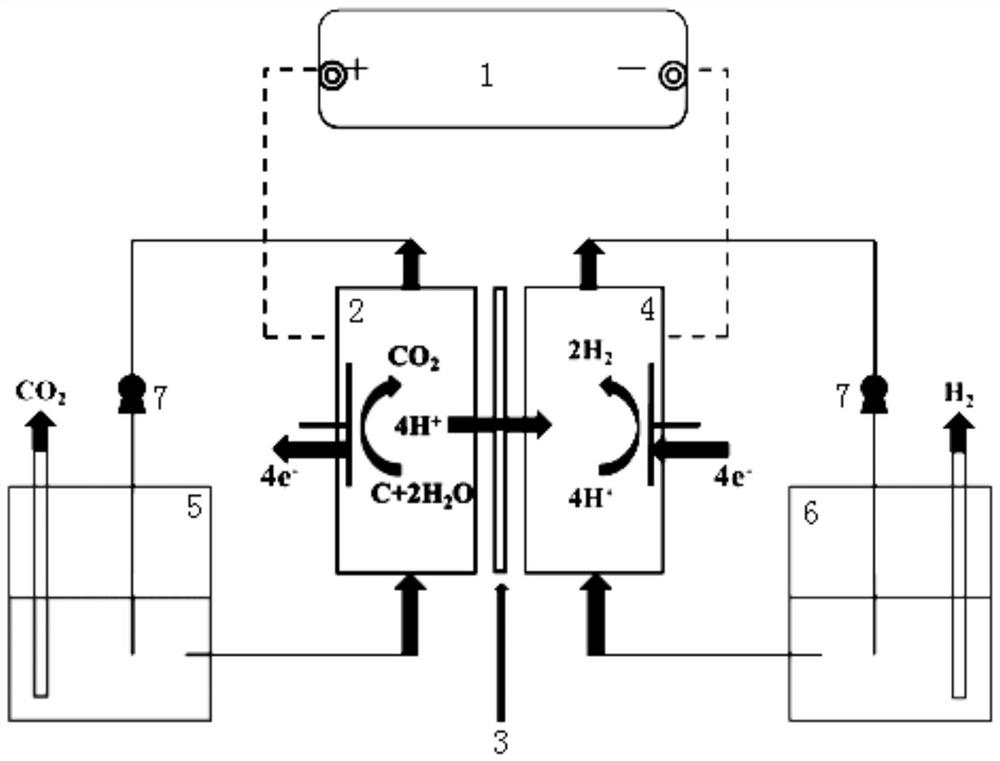
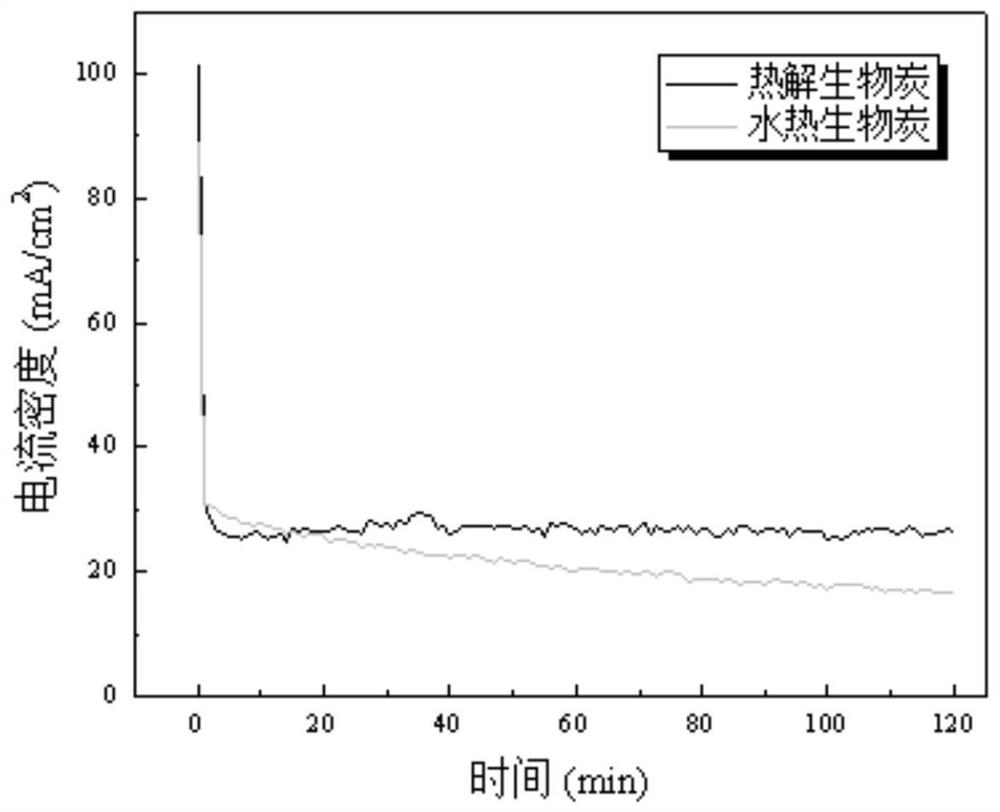
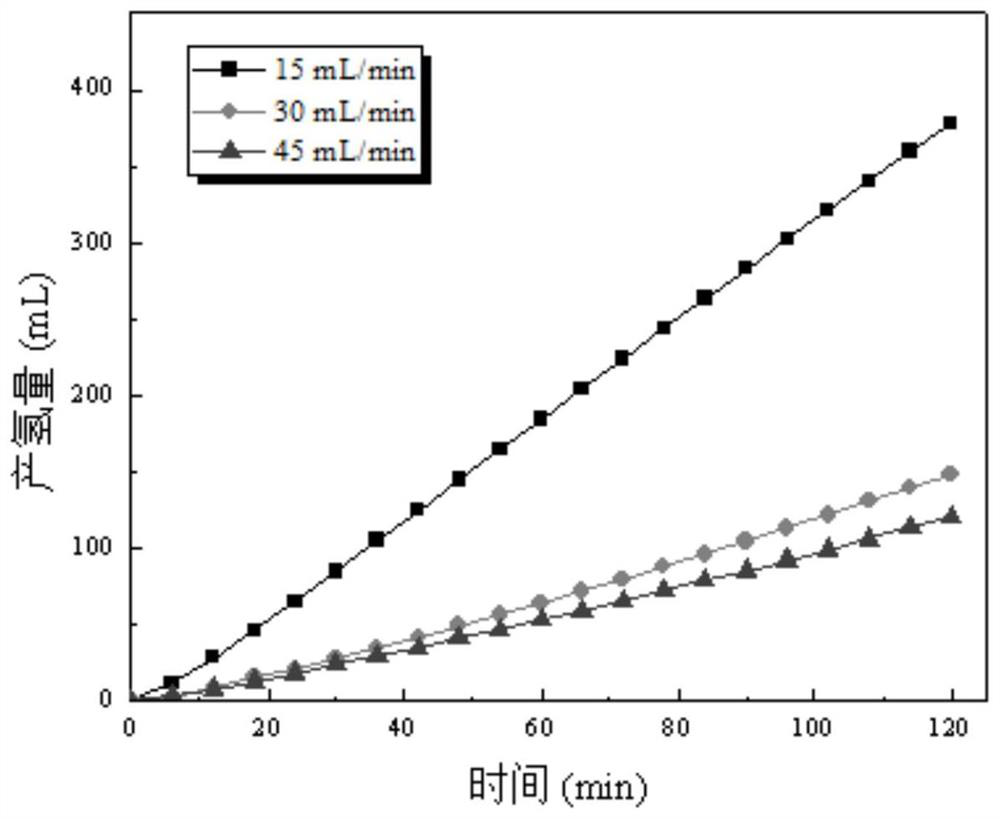
[0025] Pyrolysis and carbonization of rice husk at 600°C to produce pyrolysis biochar, configure H 2 SO 4 Biochar slurry with an electrolyte concentration of 1mol / L and a biochar slurry concentration of 0.01g / mL; the biochar was impregnated with KOH solution and activated by pyrolysis to form porous carbon, and then mixed with H 2 PtCl 6 ·6H 2 O is impregnated and reduced to uniformly load Pt on the surface of porous carbon to form a 20wt% Pt / C electrocatalyst; 2 After hot pressing with carbon paper and Nafion 117 membrane to form a membrane electrode, put it into figure 1 In the proton exchange membrane electrochemical cell; the biochar slurry and water are controlled to circulate between the proton exchange membrane electrochemical cell and the liquid storage tank at a flow rate of 30mL / min; a constant voltage of 1.3V is applied, and the cathode generates H 2 .
Embodiment 2
[0027] Pyrolysis and carbonization of rice husk at 500 °C to produce pyrolysis biochar, configure H 2 SO 4 Biochar slurry with an electrolyte concentration of 1mol / L and a biochar slurry concentration of 0.01g / mL; the biochar was impregnated with KOH solution and activated by pyrolysis to form porous carbon, and then combined with Fe(NO 3 ) 3 9H 2 Fe is evenly loaded on the surface of porous carbon by impregnation and reduction reaction to form a 20wt% Fe / C electrocatalyst; the electrocatalyst is loaded at 0.25mg / cm 2 After hot pressing with carbon paper and Nafion 117 membrane to form a membrane electrode, put it into figure 1 In the proton exchange membrane electrochemical cell; the biochar slurry and water are controlled to circulate between the proton exchange membrane electrochemical cell and the liquid storage tank at a flow rate of 15mL / min; a constant voltage of 1.3V is applied, and the cathode generates H 2 .
Embodiment 3
[0029] Peanut shells are hydrothermally carbonized at 200°C to produce hydrothermal biochar, and a biochar slurry with an HCl electrolyte concentration of 1mol / L and a biochar slurry concentration of 0.01g / mL is prepared; the biochar is impregnated with KOH solution and activated by pyrolysis to form pores char, then with H 2 PtCl 6 ·6H 2 O, Fe(NO 3 ) 3 9H 2 O uniformly supported Pt and Fe on the surface of porous carbon by impregnation reduction reaction to form a 30%wt PtFe / C electrocatalyst; the electrocatalyst was loaded at 1.8mg / cm 2 After hot pressing with carbon paper and Nafion 115 membrane to form a membrane electrode, put it into figure 1 In the proton exchange membrane electrochemical cell; control the biochar slurry and water to circulate between the proton exchange membrane electrochemical cell and the liquid storage tank at a flow rate of 45mL / min; feed a constant current density of 30mA / cm 2 , the cathode produces H 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com