Preparation and application of quality control substances of plasma type freeze-dried powder catecholamines and metabolites
A technology of catecholamine and freeze-dried powder, which is applied in the field of medical testing, can solve the problems of instability and difficult to store for a long time, and achieves the effect of good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
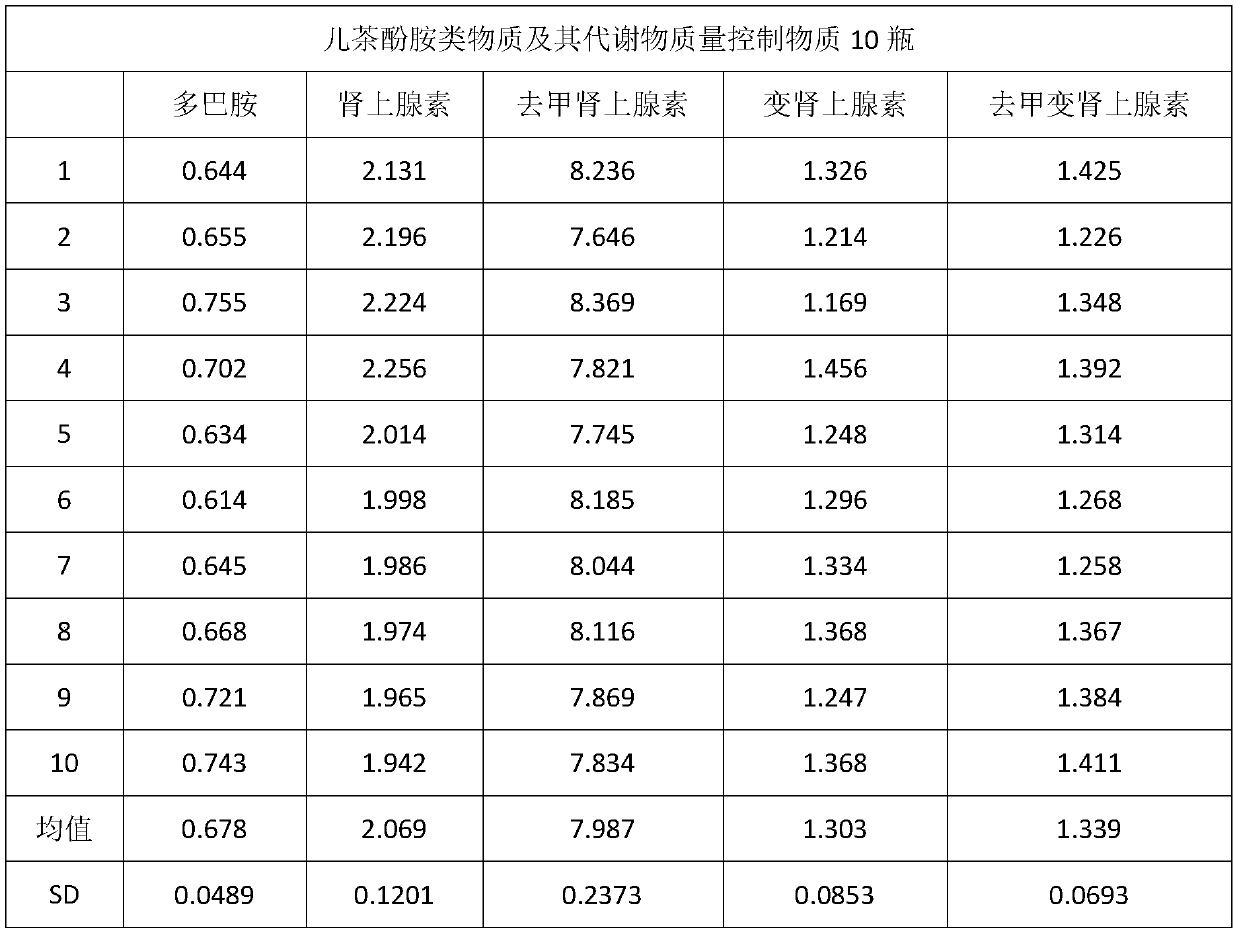
[0042] Collect the plasma without jaundice, chyle, and hemolysis, and select samples with negative infectious disease screening results (HIV, hepatitis B, syphilis, and hepatitis C) to mix together, stir evenly, and set aside.
[0043] Take 150mL of the above-mentioned mixed plasma, weigh activated carbon according to the amount of 10g / 100mL, add it to the plasma, stir slowly for 2 hours, and then filter it with 0.45μm and 0.22μm filter membranes respectively to obtain clarified plasma. After testing the catecholamines and their metabolites The content is very low and can be ignored.
[0044] Contain dopamine 0.5nmol / L, epinephrine 2.5nmol / L, norepinephrine 10nmol / L, metanephrine and noradrenaline 1.5nmol / L in the prepared quality control product, according to dopamine, epinephrine, norepinephrine Calculate the volume of the standard stock solution that needs to be added according to the concentration of the standard stock solution of metanephrine, metanephrine, and norepineph...
example 2
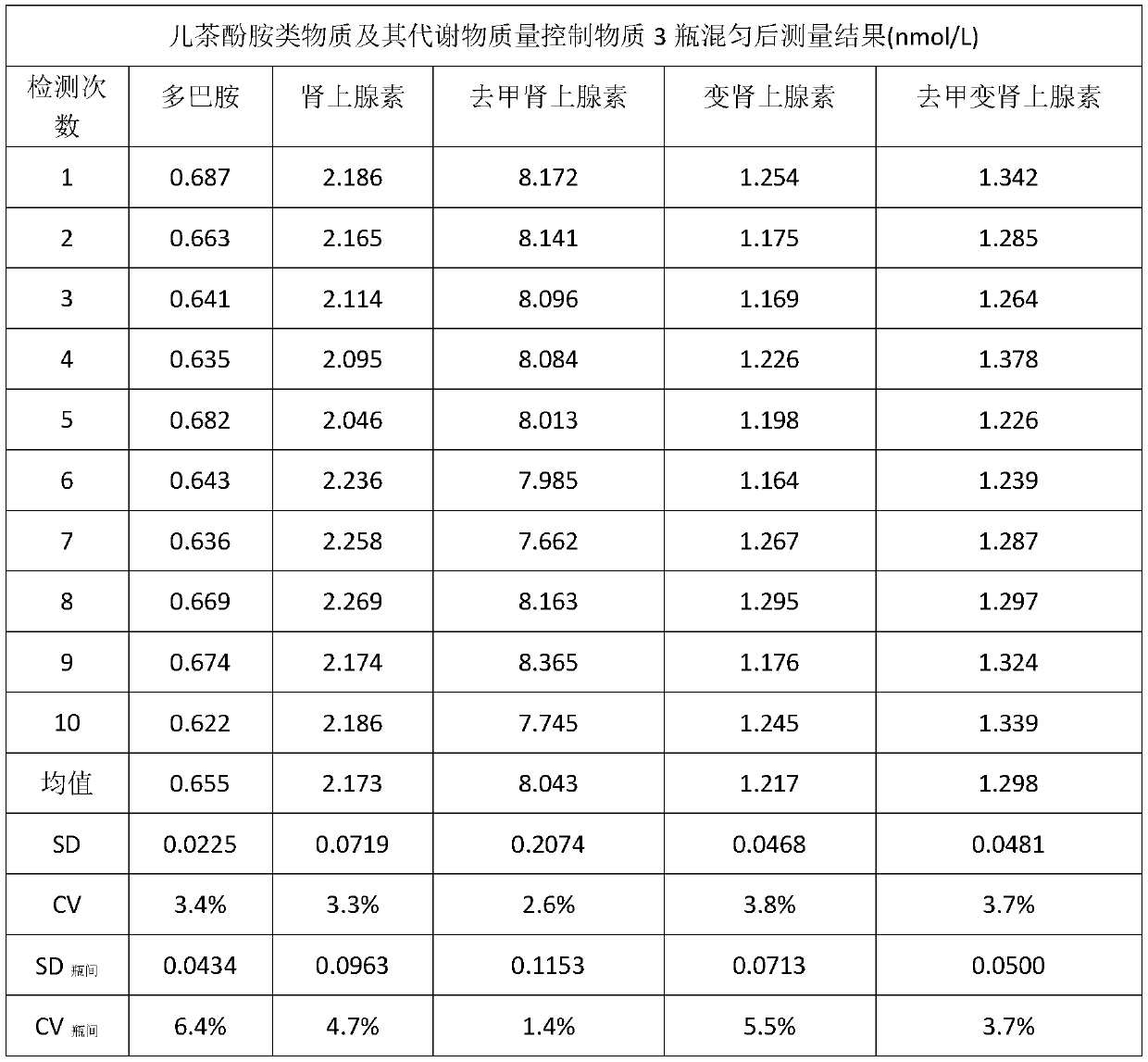
[0046] Collect the plasma without jaundice, chyle, and hemolysis, and select samples with negative infectious disease screening results (HIV, hepatitis B, syphilis, and hepatitis C) to mix together, stir evenly, and set aside.
[0047] Take 150mL of the above mixed plasma, weigh 5.0g / 100mL of activated carbon, add it to the plasma, stir slowly for 4 hours, and then filter it with 0.45μm and 0.22μm filter membranes respectively to obtain clarified plasma. After testing the catecholamines and their metabolism content is very low and can be ignored.
[0048] Contain dopamine 1.0nmol / L, epinephrine 4.0nmol / L, norepinephrine 20nmol / L, metanephrine and noradrenaline 2.0nmol / L in the prepared quality control product, according to dopamine, epinephrine, norepinephrine Calculate the volume of the standard stock solution that needs to be added according to the concentration of the standard stock solution, plasma volume (according to 100mL) and quality control substance, add the processe...
example 3
[0050] Collect anicteric, chyle, and hemolyzed plasma, and select samples with negative infectious disease screening results (HIV, hepatitis B, syphilis, and hepatitis C) to mix together, stir evenly, and set aside.
[0051] Take 150mL of the above-mentioned mixed plasma, weigh 7.5g / 100mL of activated carbon, add it to the plasma, stir slowly for 3 hours, and then filter it with 0.45μm and 0.22μm filter membranes respectively to obtain clarified plasma. After testing the catecholamines and their metabolism content is very low and can be ignored.
[0052] Contain dopamine 1.5nmol / L, epinephrine 6.0nmol / L, norepinephrine 40nmol / L, metanephrine and noradrenaline 3.0nmol / L in the prepared quality control product, according to dopamine, epinephrine, norepinephrine Calculate the volume of the standard stock solution that needs to be added according to the concentration of the standard stock solution, plasma volume (according to 100mL) and quality control substance, add the processed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com