Phytase mutant
A technology of mutants and phytase, applied in the direction of enzymes, hydrolytic enzymes, plant gene improvement, etc., can solve the problems of low enzyme activity level and inability to meet the requirements of aquaculture, and achieve broad prospects, improved tolerance, Effect of heat resistance improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Screening of Phytase Mutants Tolerant to Neutral pH Environment
[0041] 1.1 Mutant screening
[0042]In order to improve the tolerance of Escherichia coli-derived phytase PHY-M (the amino acid sequence is SEQ ID NO: 1, and its coding nucleotide sequence is SEQ ID NO: 2) under neutral pH conditions, the applicant first entrusted Shanghai Jierui Bioengineering Co., Ltd. optimized and synthesized the above sequence according to the codon preference of Pichia pastoris, and added EcoRI and NotI restriction sites at the 5' and 3' ends of the synthetic sequence, respectively.
[0043] The protein structure analysis of the PHY-M gene synthesized above shows that the protein has two structural domains: 134 amino acid residues at the N-terminus and 152 amino acid residues at the C-terminus together form domain 1, and the remaining 124 amino acid residues in the middle form the structure Domain 2, the conserved sequence and active center are all located in domain 1, an...
Embodiment 2
[0081] Example 2 Screening of high temperature resistant phytase mutants
[0082] 2.1 Mutant screening
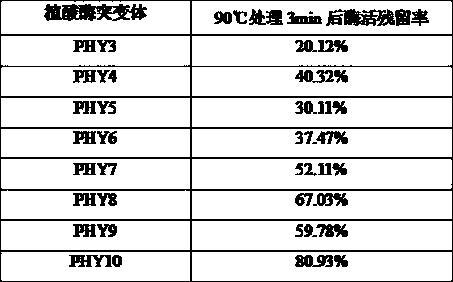
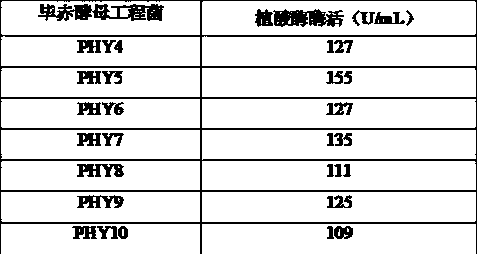
[0083] In order to improve the heat resistance of the phytase mutant PHY3 (its amino acid sequence is SEQ ID NO: 5, and its coding nucleotide sequence is SEQ ID NO: 6), the applicant further carried out a large number of mutations on the enzyme through directed evolution technology filter.
[0084]Using the PHY3 gene as a template, using the primers PHY-M-F1 and PHY-M-R1 described in Example 1, PCR amplification was performed with the GeneMorphII Random Mutation PCR Kit (Stratagene), and the PCR product was recovered from the gel, EcoRI, Not I After enzyme digestion, it was ligated with the pET21a vector after the same enzyme digestion, transformed into Escherichia coli BL21(DE3), spread on LB+Amp plate, and cultured upside down at 37°C. After the transformants appeared, pick them one by one with a toothpick. In a 96-well plate, add 150 ul of LB+Amp medium containing 0.1m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com