Pyrimidine derivative with anticancer effect
A use and drug technology, applied in the field of pyrimidine derivatives, can solve the problems of large side effects, poor selectivity, and troubled clinical ovarian cancer treatment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1 Preparation of 2-(2-(2-hydroxybenzylidene)hydrazino)pyrimidine-4-ol (compound 1)
[0072]
[0073] Add 2-hydrazinopyrimidin-4-ol (53mg, 0.5mmol) and 2-hydroxybenzaldehyde (106μL, 1mmol) and 5mL methanol into a 25mL round bottom flask, and reflux overnight at 65°C. The next day, it was cooled to room temperature, filtered with suction, and the filter cake was washed successively with 10 mL of absolute ethanol and 20 mL of absolute ether, and dried to obtain 98 mg of off-white solid, with a yield of 85.2%.
[0074] 1 H NMR (400MHz, DMSO-d 6 )δ11.61(s,2H),9.01(s,1H),7.70(dd,J=7.7,1.5Hz,1H),7.43–7.37(m,2H),6.98(t,J=7.6Hz,3H ),5.81(d,J=7.6Hz,1H).
[0075] ESI-ms(m / z):231.1[M+H] +
[0076] According to the preparation method of compound 1, compound 2-compound 24 can be prepared by reacting different 2-hydrazinopyrimidine derivatives with corresponding aldehydes or ketones, and its structure and characterization data are shown in Table 1:
[0077] The struct...
Embodiment 2
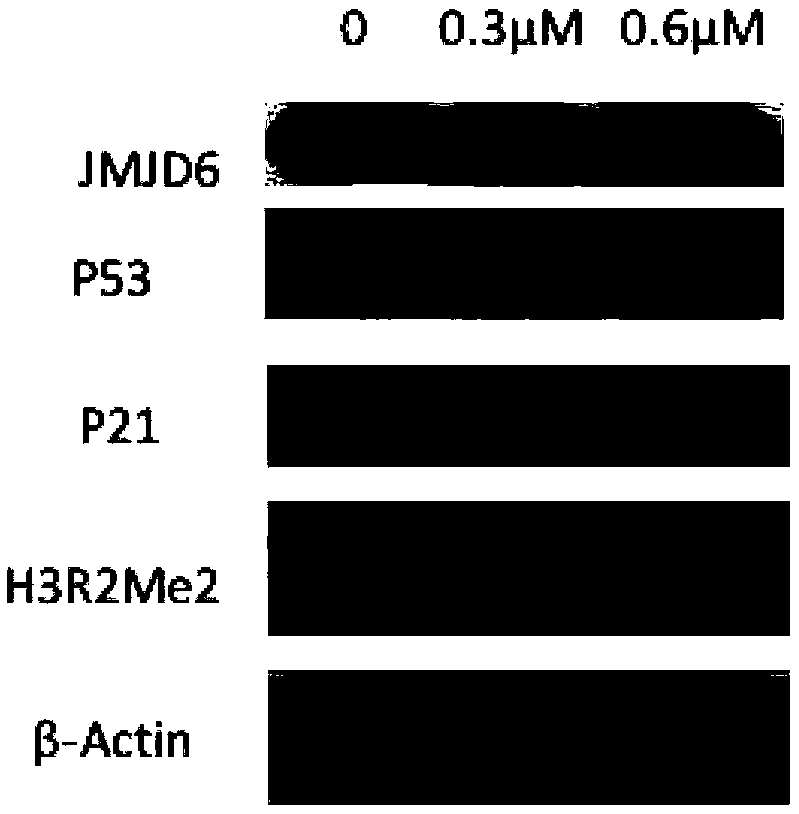
[0082] Example 2 The inhibitory effect of the compound of the present invention on histone demethylase
[0083] Experiment purpose: To detect the inhibitory activity of the compound of the present invention on histone demethylase in vitro, using Succinate-Glo TM The JmjC Demethylase / Hydroxylase Assay (Promega) kit tests the in vitro inhibitory activity of compounds on arginine demethylase JMJD6.
[0084] Experimental principle: During histone demethylation, the cofactor α-ketoglutarate is reduced to succinate. If histone demethylase activity is inhibited, α-ketoglutarate will not be reduced. Therefore, the activity of histone demethylase can be reflected indirectly by measuring the concentration of succinic acid.
[0085] Experimental method: In a 384-well plate, add 2.5 μL of a certain concentration of small molecule solution and 2.5 μL of JMJD6 protein solution. The concentration of JMJD6 protein solution is 2.2 mg / mL. After incubation at room temperature for 10 minutes, a...
Embodiment 3
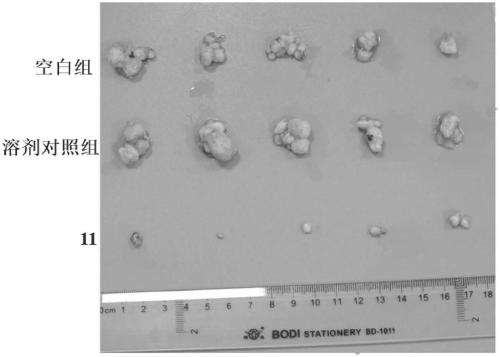
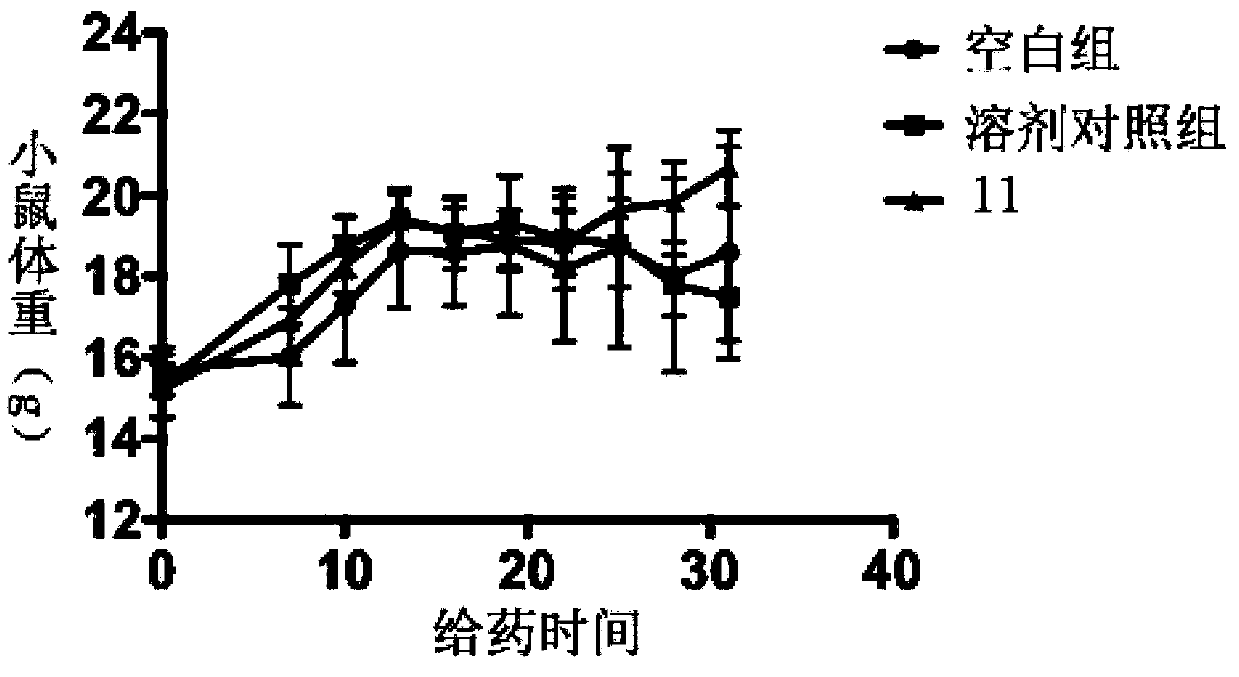
[0093] Example 3 Compound 11 Inhibitory Effect on Human Tumor Cell Proliferation
[0094] Experimental purpose: To detect the inhibitory activity of the compound of the present invention on human tumor cell proliferation in vitro, and to test the inhibitory activity of the tested compound on different tumor cell lines by using the Cell Counting kit-8 (CCK8) method. IC 50 (half inhibitory concentration). IC 50 The value can be obtained by calculating the inhibitory rate of the test compound on tumor cells at a series of different concentrations.
[0095] Experimental principle: WST-8(2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfobenzene)-2H-tetrazole Monosodium salt) in the presence of the electron coupling carrier 1-Methoxy PMS, can be reduced by the dehydrogenase in the mitochondria to generate a highly water-soluble orange-yellow formazan product (formazan), and the depth of the color is proportional to the proliferation of the cells. Inversely proportional...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com